Authors
P. Fernández-Guzmán, I. Salvador, V. Navarro, A. Aguilera, G. Pujadas, J. Castellví, D. Medina-Gil, S. Mallorquí-Alcalá, M. Erguin, P. Abrisqueta, Á. Serna, F. Bosch, M. Crespo.
Background
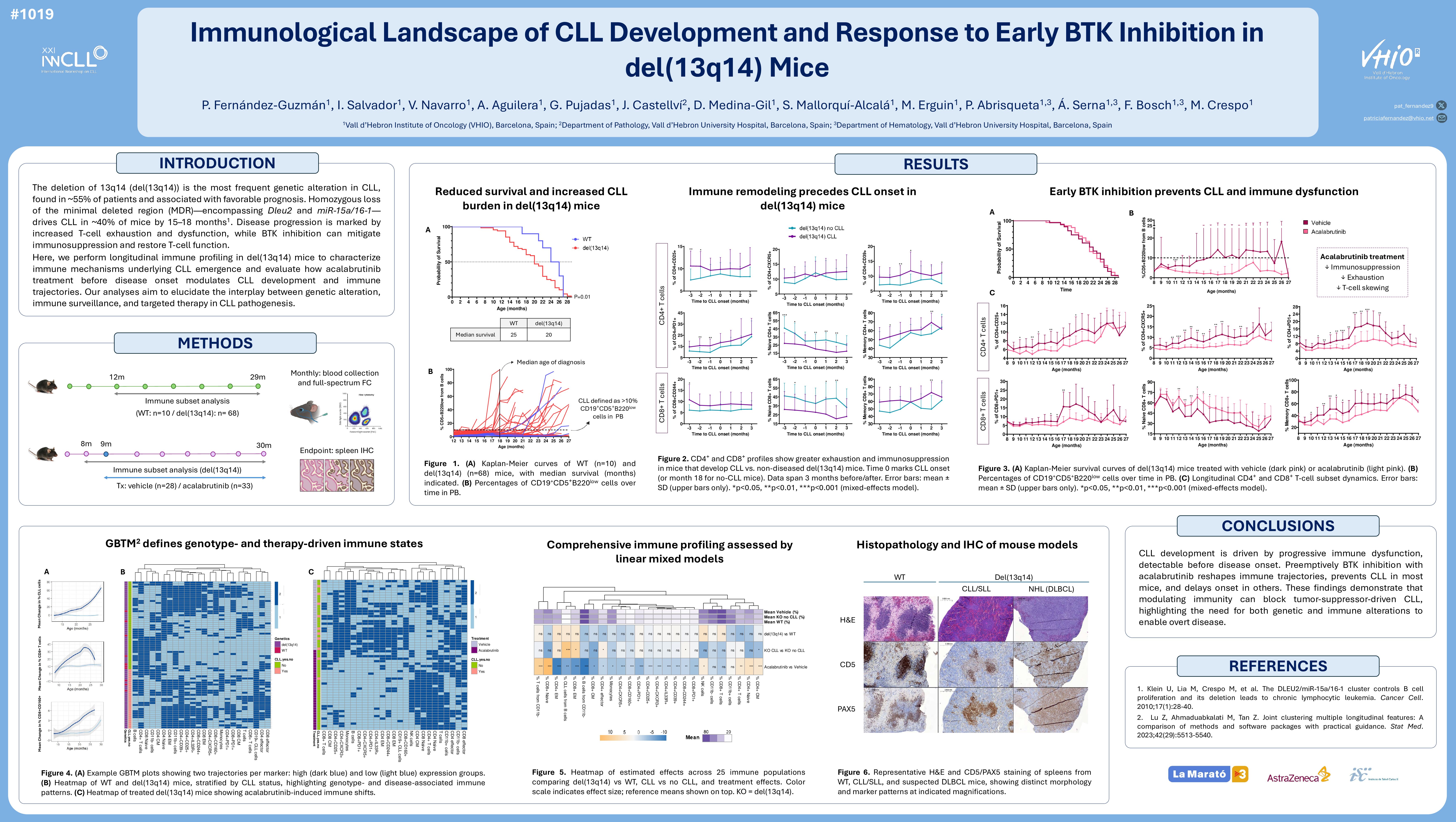
The deletion of 13q14 (del(13q14)) is the most prevalent genetic alteration in Chronic Lymphocytic Leukemia (CLL), affecting approximately 55% of patients and associated with favorable prognosis. Deletion of the minimal deleted region (MDR) within del(13q14), encompassing the DLEU2 gene and the miR-15a/16-1 cluster, leads to CLL development in around 40% of mice by 15–18 months (Klein et al., 2010). In patients, clinical progression is marked by increasing T-cell exhaustion and dysfunction. BTK inhibition can reduce immunosuppression and restore T-cell function.
Based on these observations, we hypothesized that immune surveillance initially restrains CLL in del(13q14) mice, but progressive immune dysfunction allows disease emergence. We further postulated that early BTK inhibition could modulate immune– CLL interactions, preventing immune escape and overt disease.
Our objectives were to elucidate the immune mechanisms driving CLL progression through longitudinal immune profiling, and to assess how early therapeutic intervention with acalabrutinib alters CLL development and immune trajectories in del(13q14) mice.
Methods
Two murine cohorts were established: one with 62 del(13q14) mice and 10 wild-type (WT) controls, with monthly blood sampling from 12 months of age to endpoint; and another with 61 del(13q14) mice randomized to acalabrutinib (n=28, 25 mg/kg daily) or vehicle (n=33) from 9 months of age (prior to CLL onset), with sampling beginning at 8 months. All samples were analyzed by spectral flow cytometry to monitor immune dynamics. Longitudinal changes in CLL and immune subsets (T, NK, monocytes) were assessed using linear mixed models. Generalized Bayesian trajectory modeling (GBTM) clustered mice by immune trajectory for each population. CLL was defined as >10% CD5⁺B220^low among CD19⁺ cells.
Results
Untreated del(13q14) mice had shorter survival than WT (median: 20 vs. 25 months, p=0.01). CLL developed in 42% of del(13q14) mice (median onset: 18 months), with leukemic burden increasing over time: 4.5% at 12 months, 11.9% at 20 months, and 27% at 25 months. Although overall T, NK, and monocyte proportions were similar between WT and del(13q14) mice, those developing CLL showed greater increases in regulatory T cells (Tregs; CD4⁺CD25⁺ and CD4⁺CD39⁺), immunosuppressive CD4⁺IL33R⁺ cells, T follicular helper cells (Tfh; CD4⁺CXCR5⁺), and exhausted CD4⁺PD1⁺ T cells compared to non-diseased del(13q14) mice. A more pronounced decrease in CD4⁺ naïve T cells, along with an increase in effector memory (EM) CD4⁺ T cells, were also observed in CLL-developing mice from the start of the follow-up period. Similarly, CD8⁺CD244⁺ and CD8⁺PD1⁺ T cells increased in these mice, with early loss of naïve CD8⁺ T cells and EM expansion.
GBTM identified two major immune trajectory clusters per variable. Over 70% of CLL-developing mice followed trajectories with increasing Tregs and a shift from naïve to EM CD8+ T cells (–55% naïve, +20% memory) by endpoint.
Acalabrutinib did not significantly affect overall survival but reduced CLL incidence (6% vs. 28% in vehicle-treated mice). Mean CLL cell frequencies remained lower with acalabrutinib: 2% vs. 6% at 12 months; 2% vs. 11% at 19 months; and 3% vs. 13% at 24 months. Mice receiving vehicle showed significantly higher increases in Tregs, Tfh, IL33R⁺, and PD1⁺ CD4⁺ T cells (p < 0.05 in all populations during >50% of follow-up). They also exhibited a greater decline in CD4⁺ naïve T cells, with marked expansion of central memory (CM) and especially EM T cells (p < 0.01 from months 12–26). No major differences in CD8⁺CD244⁺ and CD8⁺CD160⁺ T cells were observed between groups, but CD8⁺PD1⁺ levels were significantly higher in vehicle-treated mice from months 17–22. CD8⁺ naïve T cells declined more rapidly in vehicle-treated mice (p < 0.05 from months 11–26), with a significant increase in CD8⁺ memory cells.
Consistently, GBTM showed that 57% of vehicle-treated mice followed trajectories with increasing regulatory/exhausted T cells, ~50% reduction in naïve CD8⁺, and +20% increase in EM CD8⁺ cells by endpoint. In contrast, >70% of acalabrutinib-treated mice showed only modest increases in these subsets, and a limited decline in naïve CD8⁺ cells (–15%).
Conclusion
Progressive immune dysfunction facilitates CLL development in del(13q14) mice. Early acalabrutinib treatment prevents CLL in most mice, delays onset in others, and modifies the immune landscape. Although early BTK inhibition has not yet improved OS in patients diagnosed with CLL, our findings suggest that early immunomodulatory strategies could prevent or delay MBL/CLL progression.
Keywords : immune landscape, mouse models, BTK inhibition
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: Fundació La Marató de TV3, Astrazeneca