Authors
AliciaSerrano-Alcalá, LauraVentura-López, AzaharaFuentres-Trillo, BlancaFerrer-Lores, FelipeJavierChaves, MaríaJoséTerol-Casterá.
Introduction
Prognostic stratification in Chronic Lymphocytic Leukemia (CLL) relies on the CLL-IPI index, which incorporates clinical, cytogenetic, and biological markers, notably IGHV mutational status and TP53 alterations. Over the past decade, next-generation sequencing (NGS) has revealed key genomic, epigenomic, and transcriptomic features of CLL, identifying alterations linked to disease onset, progression, and therapy resistance. These advances have supported the development of refined prognostic tools and targeted therapies. However, CLL remains a paradigm of cancer evolution: despite effective treatments, clonal progression and relapse are common. Clonal evolution post-immunochemotherapy (ICT) is typical, and resistance to targeted agents like Ibrutinib (IB) arises through tumor adaptation. The prognostic and predictive significance of clinical and clonal parameters in guiding therapy remains unclear. This study aims to evaluate the impact of these variables on clonal evolution in CLL.
Patients and methods
A retrospective observational study was conducted, including 96 untreated patients diagnosed with CLL/CLL-like (MBL) between 1999-2024. Key clinical and biological disease characteristics were recorded, along with dates of progression, treatment initiation, and response. Mutational status of TP53, SF3B1, BIRC3, NOTCH1, MYD88, XPO1, EGR2, POT1, NFKBIE, ATM, and FBXW7 was assessed in baseline samples and 302 follow-up samples using four independents targeted NGS panels based on Illumina paired-end short-read (150×2) sequencing technology. The IBR-R panel was designed and validated using Illumina short-read NGS for detecting resistance-associated mutations in BTK and PLCγ2 in patients treated with IB.
Results
In the baseline cohort, missense mutations were predominant in SF3B1, TP53, XPO1, and ATM, while NOTCH1 and BIRC3 mutations were primarily frameshift. Clonal and subclonal variants (VAF < 10%) shared similar profiles, except in ATM, MYD88, and NOTCH1, where only clonal variants were identified. Convergent evolution was observed in 13 patients across TP53, SF3B1, ATM, XPO1, and BIRC3.
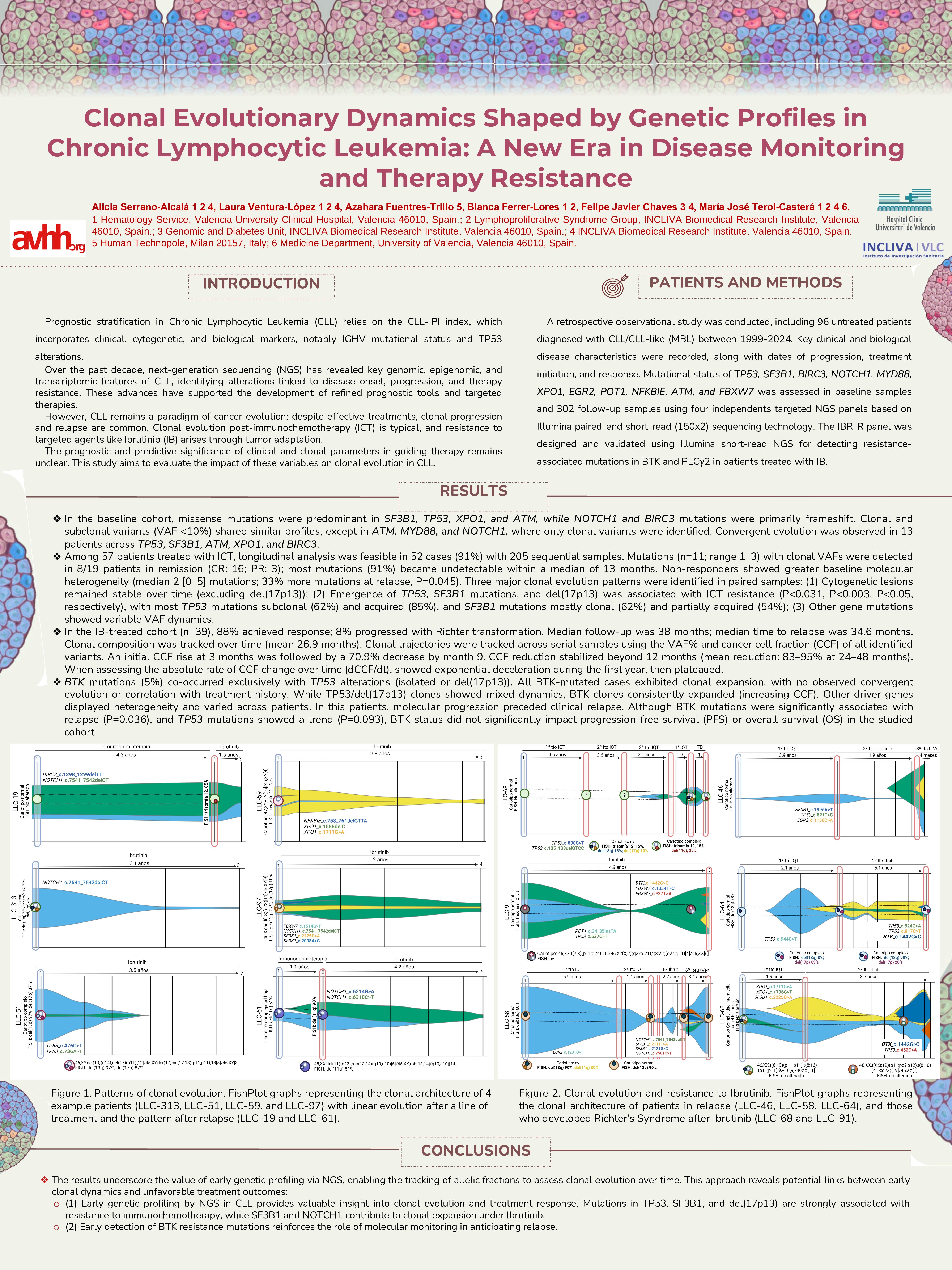
Among 57 patients treated with ICT, longitudinal analysis was feasible in 52 cases (91%) with 205 sequential samples. Mutations (n=11; range 1–3) with clonal VAFs were detected in 8/19 patients in remission (CR: 16; PR: 3); most mutations (91%) became undetectable within a median of 13 months. Non-responders showed greater baseline molecular heterogeneity (median 2 [0–5] mutations; 33% more mutations at relapse, P=0.045). Three major clonal evolution patterns were identified in paired samples: (1) Cytogenetic lesions remained stable over time (excluding del(17p13)); (2) Emergence of TP53, SF3B1 mutations, and del(17p13) was associated with ICT resistance (P < 0.031, P< 0.003, P< 0.05, respectively), with most TP53 mutations subclonal (62%) and acquired (85%), and SF3B1 mutations mostly clonal (62%) and partially acquired (54%); (3) Other gene mutations showed variable VAF dynamics.
In the IB-treated cohort (n=39), 88% achieved response; 8% progressed with Richter transformation. Median follow-up was 38 months; median time to relapse was 34.6 months. Clonal composition was tracked over time (mean 26.9 months). Clonal trajectories were tracked across serial samples using the VAF% and cancer cell fraction (CCF) of all identified variants. An initial CCF rise at 3 months was followed by a 70.9% decrease by month 9. CCF reduction stabilized beyond 12 months (mean reduction: 83–95% at 24–48 months). When assessing the absolute rate of CCF change over time (dCCF/dt), showed exponential deceleration during the first year, then plateaued.
BTK mutations (5%) co-occurred exclusively with TP53 alterations (isolated or del(17p13)). All BTK-mutated cases exhibited clonal expansion, with no observed convergent evolution or correlation with treatment history. While TP53/del(17p13) clones showed mixed dynamics, BTK clones consistently expanded (increasing CCF). Other driver genes displayed heterogeneity and varied across patients. In this patients, molecular progression preceded clinical relapse. Although BTK mutations were significantly associated with relapse (P=0.036), and TP53 mutations showed a trend (P=0.093), BTK status did not significantly impact progression-free survival (PFS) or overall survival (OS) in the studied cohort
Conclusions
The results underscore the value of early genetic profiling via NGS, enabling the tracking of allelic fractions to assess clonal evolution over time. This reveals potential links between early clonal dynamics and unfavorable treatment outcomes: (1) Early genetic profiling by NGS in CLL provides valuable insight into clonal evolution and treatment response. Mutations in TP53, SF3B1, and del(17p13) are strongly associated with resistance to immunochemotherapy, while SF3B1 and NOTCH1 contribute to clonal expansion under Ibrutinib. (2) Early detection of BTK resistance mutations reinforces the role of molecular monitoring in anticipating relapse.
Keywords : Clonal Evolution, Next-Generation Sequencing (NGS), Molecular Monitoring
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: Lymphoproliferative Syndrome Group, INCLIVA Biomedical Research Institute. Hematology Service, Valencia University Clinical Hospital