Authors
Hao Guxi , Yongyi Yuan, Siming Zheng, Keshu Zhou
Background
Chronic lymphocytic leukemia (CLL) is an uncurable hematologic malignancy marked by metabolic reprogramming and immune evasion. SF3B1 mutations, present in 10-15% CLL, are associated with poor prognosis due to their disruption of RNA splicing. While SF3B1 mutations impact numerous cellular processes, their effects on tumor metabolism and immune modulation remain incompletely understood. SAT1 (Spermidine/spermine N1-acetyltransferase 1) plays a crucial role in polyamine metabolism, which is essential for cell growth and survival. This study investigates how SF3B1 K700E mutation regulates SAT1 expression, leading to metabolic reprogramming and immune modulation in CLL.
Results
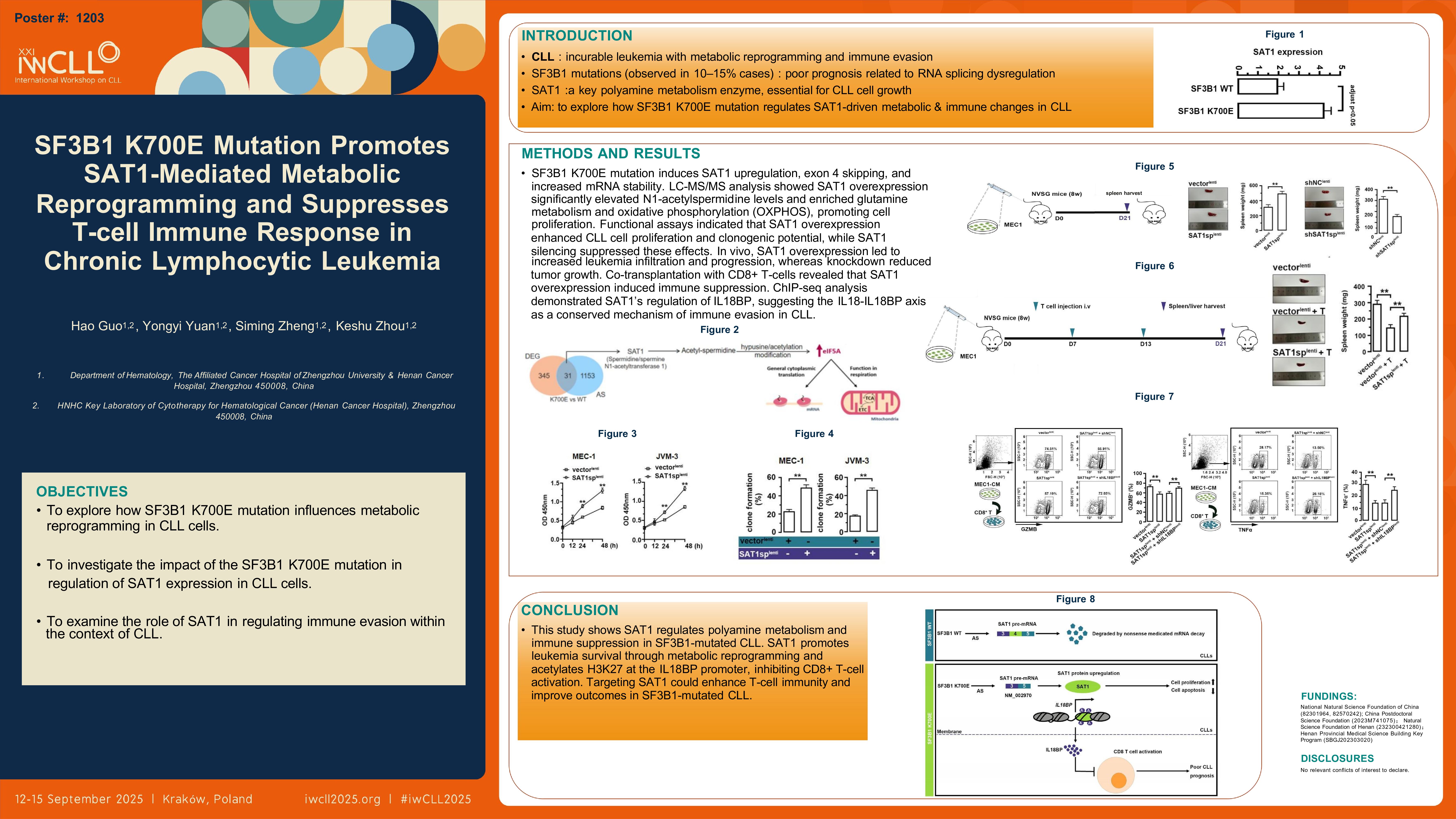
To explore the effects of SF3B1 K700E mutation on metabolic gene expression , we conducted RNA profiling on MEC-1 cells expressing SF3B1 K700E and primary CLL samples from SF3B1-mutated patients. Integrated RNA expression and splicing analysis revealed SAT1 upregulation in SF3B1-mutated samples, with exon 4 skipping and increased mRNA stability. We then used LC-MS/MS-based targeted metabolomics to assess the impact of SAT1 overexpression (SAT1-OE) on polyamine metabolism. The results revealed a significant increase in N1-acetylspermidine levels in SAT1-OE CLL cells, consistent with upregulation in SF3B1-mutated cells compared to wild-type (WT) controls. Additionally, untargeted metabolomics analysis identified glutamine metabolism and oxidative phosphorylation (OXPHOS) metabolism as significantly enriched in SAT1-OE CLL cells, suggesting a shift in metabolic pathways that support cell survival and proliferation.In functional assays, SAT1 overexpression in MEC-1 and JVM-3 cells, as well as primary CLL cells, enhanced cell proliferation and clonogenic potential, while SAT1 silencing suppressed these effects. We next performed in vivo studies using NSG mice to evaluate SAT1’s impact on leukmia infiltration and progression. SAT1 overexpression in CLL cells led to increased leukemia burden, with significantly larger spleens and livers.In contrast, SAT1 knockdown decreased tumor growth and infiltration. In co-transplantation model with CD8+ T-cells, SAT1 overexpression increased leukemic infiltration and induced immune suppression, evidenced by decreased T-cell number and reduced IFN-γ production. In contrast, SAT1 knockdown reduced leukemia burden, restored CD8+ T-cell activation, indicating improved immune responses. Further, we performed ChIP-seq combined with RNA-seq to examine the role of SAT1 in epigenetic regulation. We identified H3K27 acetylation enrichment at the IL18BP promoter, which facilitated IL18BP transactivation in SAT1-overexpressing MEC-1 cells. IL18BP levels were significantly higher in the culture supernatants of SAT1-OE CLL cells, confirming its role in immune suppression within the tumor microenvironment (TME). Interestingly, SAT1 was found to non-canonically acetylate H3K27 domains in multiple mitosis-regulating genes in ovarian cancer (Zheng et al., Nature Communications 2025). This finding suggests that SAT1-mediated regulation of the IL18-IL18BP axis may serve as a conserved oncogenic mechanism that supports immune evasion in CLL.
Conclusion
This study provides evidence for a dual mechanism by which SAT1 regulates polyamine metabolism and immune suppression in SF3B1-mutated CLL. SAT1 enhances polyamine catabolism and supports leukemia survival through metabolic reprogramming. Additionally, SAT1 probably acetylates H3K27 at the IL18BP promoter, increasing IL18BP expression and inhibiting CD8+ T-cell activation, leading to immune evasion. These findings suggest that targeting SAT1 could provide a therapeutic strategy to enhance T-cell-mediated immunity and improve outcomes in SF3B1-mutated CLL.
Keywords : SF3B1 mutation, SAT1, Chronic lymphocytic leukemia
Please indicate how this research was funded. : This work was supported by the National Natural Science Foundation of China (82301964), the China Postdoctoral Science Foundation (2023M741075), the Natural Science Foundation of Henan (232300421280), and the Henan Provincial Medical Science Building Key Program (SBGJ202303020).
Please indicate the name of the funding organization.: National Natural Science Foundation of China
China Postdoctoral Science Foundation
Natural Science Foundation of Henan
Henan Provincial Medical Science Building Key Program