Authors
Santiago Rodríguez Zraquia, Eugenia Payque, Rita Uria, Jorge Souto, Diego Álvarez, Ana Inés Landoni, Victoria Remedi, Carolina Oliver, Sabrina Ranero, Victoria Irigoin, Virginia Lema, Marcelo Hill, Florencia Festari, Marcelo Navarrete and Pablo Oppezzo.
Chronic lymphocytic leukemia (CLL) cell proliferation and survival are supported by tumor microenvironment (TME) interactions, antigen/autoantigen (Ag/autoAg) stimulation, and/or autonomous signalling, among others (Kipps et al., 2017). B-cells are typically activated in the germinal center upon B-cell receptor (BCR)-Ag stimulation. B-cells interact with T-follicular helper cells, inducing upregulation of activation-induced cytidine deaminase (AID), a mutagenic enzyme essential to initiate somatic hypermutation and class-switch recombination. AID preferentially mutates Ig genes (VDJ or pre-switched regions); however, uncontrolled expression of this enzyme could result in “off-target” mutations in proto/oncogenes, originating cancer development and/or tumor progression (Okazaki et al., J Exp Med, 2003; Morande and Yan et al., Blood, 2021). In CLL, AID expression by CLL cells with a “naïve” phenotype (CD5+/IgM+/IgD+) in unmutated patients (U-CLL) with clinical poor outcome (Oppezzo et al., Blood, 2003), as well as its role during disease progression, remain open questions.
Despite significant progress in prognostic tools, accurately predicting disease progression remains challenging in many CLL patients. To address this issue, in previous work by Souto, Landoni et al. (abstract #1144, iwCLL_2023), we studied AID expression in the peripheral blood of U-CLL patients and analyzed its association with immunoglobulin heavy chain gene (IGHV) status/usage and time to first treatment (TTFT). This analysis led to the identification of a novel CLL subgroup, Subgroup_1 (Sb_1), characterized by high AID expression, specific IGHV rearrangements (1-69, 1-02, 3-30, and 4-39), and shorter TTFT. The second subgroup (Sb_2) is characterized by expression of the same IGHV rearrangements but low levels of AID and longer TTFT.
Our central hypothesis is that the poor clinical outcome observed in Sb_1 corresponds to an activated tumour microenvironment (aTME) that enhances AID expression through continuous stimulation by an unknown antigen or autoantigen. This is supported by the biased usage of BCR variable regions in these patients and by previous WES data showing canonical AID off-target mutations in key leukemic drivers (Morande and Yan et al., Blood, 2021). We believe that some of these AID mutations confer a greater tumour “fitness” of CLL cells which explain at least in part, the poorest clinical outcome of this subgroup.
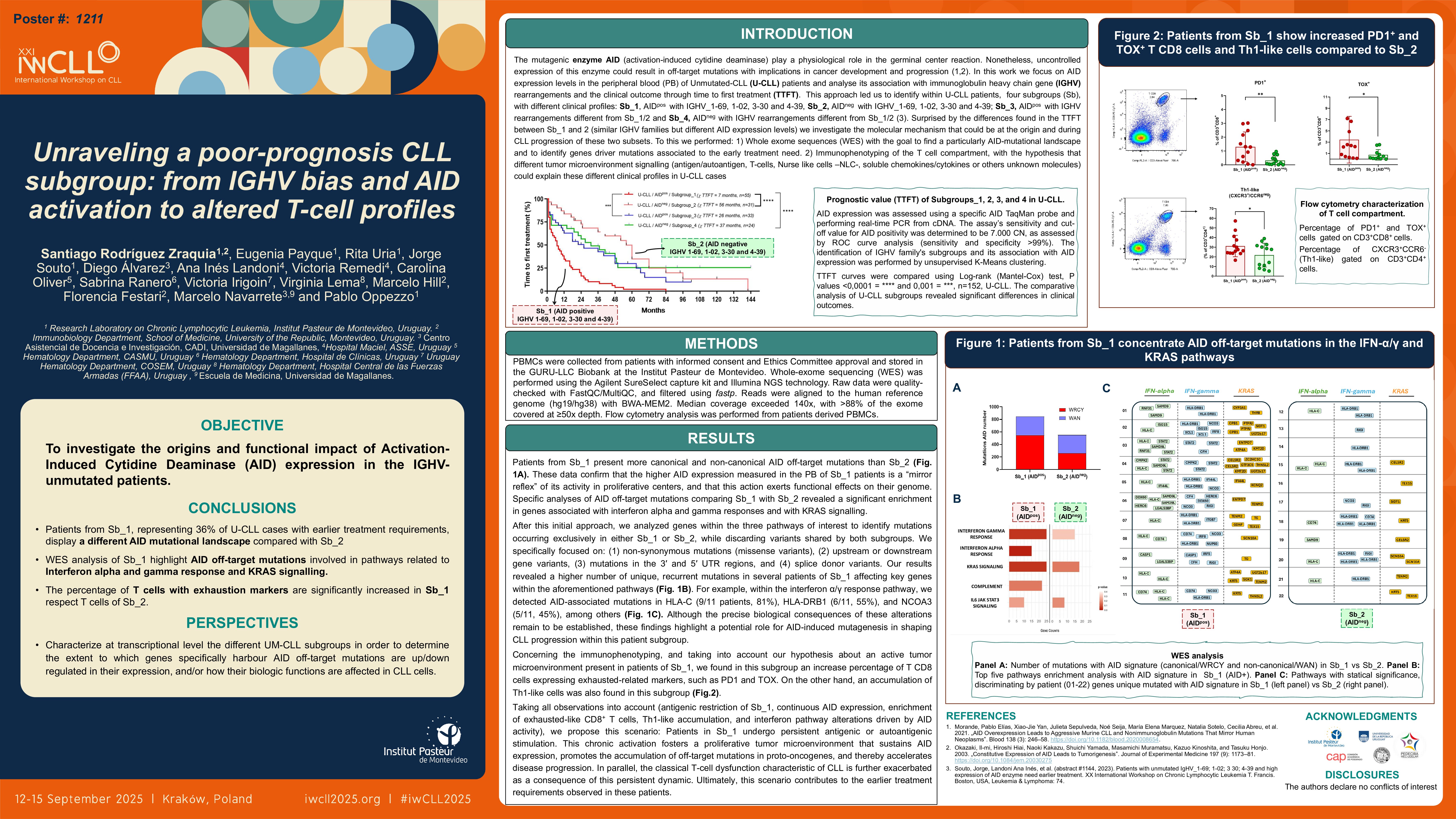
To better characterize the TME in these two subgroups and considering the relevance of the T-cell compartment in CLL evolution, we analysed the phenotypic profile of the T-cells in Sb_1 and Sb_2. To this, PBMCs were collected from patients with informed consent and Ethics Committee approval and stored in the GURU-LLC Biobank at the Institut Pasteur de Montevideo. Flow cytometry was used to characterize both the tumor clone and T-cell populations.
Our results revealed a significantly higher percentage of exhausted-like T cells in Sb_1 compared to Sb_2. This was evidenced by increased CD3+/CD8+/PD1+ cells (mean difference: 7.1; means 8.29 vs. 1.13; p = 0.0264, n = 18) and CD3+/CD8+/TOX+ cells (mean difference: 5.98; means 7.75 vs. 1.77; p = 0.0445, n = 18).
For CD4+ T-helper cells, subsets were defined by CXCR3 and CCR6 expression: Th1-like (CXCR3+ CCR6−), Th2-like (CXCR3− CCR6−), Th17-like (CXCR3− CCR6+), and CXCR3+/CCR6+ (Th0 or Tregs). Interestingly, Sb_1 showed a decrease in Th17-like cells (mean difference: 3.65; means 8.11 vs. 11.77; p = 0.0535) and a significantly reduced Th2/Th1 ratio (mean difference: 3.13; means 1.54 vs. 4.68; p = 0.0370).
Persistent antigen stimulation in CLL contributes to T-cell dysfunction and exhaustion, correlating with poor outcomes (Jimenez et al., Biomarker Research, 2021). Our finding that Sb_1 is enriched in exhausted-like CD8+ T cells suggest a more immunosuppressive TME in this subgroup, supporting our hypothesis. Although the role of T-helper cells in CLL remains debated, Th1/IFN-γ+ cells may promote disease progression by increasing CD38 expression and inhibiting apoptosis in leukemic cells (Bürgler et al., JOI, 2015; Roessner et al., BJH, 2020). The lower Th2/Th1 ratio in Sb_1 may reflect increased IFN-γ activity, potentially contributing to early progression. Despite it is an interesting observation in the context of the earlier progression of Sb_1, more studies will be performed in this line.
Altogether, these results suggest that Sb_1, representing 36% of U-CLL cases, is characterized by a distinct T-cell profile associated with an adverse TME. Further studies are needed to explore the contributions of IGHV rearrangements, AID expression, and T-cell dysfunction to CLL progression and therapy resistance.
Keywords : CLL AID T-Cells
Please indicate how this research was funded. : Fondo Clemente Estable FCE_1_2021_1_166493
Becas de apoyo a docentes para estudios de posgrado en la Udelar
Please indicate the name of the funding organization.: ANII
CAP