Authors
S. Mallorquí-Alcalá, I. Salvador, C. Palacio, M. Bastos-Boente, M. Alcoceba, C. de Ramón, A. Navarro, D. Medina-Gil, P. Fernández-Guzmán, M. Erguin, Á. Serna, P. Abrisqueta, F. Bosch, M. Crespo.
Background
CLL cells behave as regulatory B cells, altering the functionality and proportion of immune subsets, especially T cells (Mékinian et al., 2023). T lymphocytes from CLL patients exhibit dysregulated expression of activation molecules and increased expression of exhaustion markers (Riches et al., 2013). Previous evidence from our group suggests that immune dysfunction, particularly T cell exhaustion, is increased at disease progression (Jiménez et al., 2021). Therefore, we hypothesized that progressive immune dysfunction during the first months after diagnosis can identify patients at higher risk of early progression. To assess that, we longitudinally analyzed patient samples at diagnosis and six months later to correlate immune dysfunction with progression risk.
Methodology
Cryopreserved peripheral blood mononuclear cells were obtained from 34 CLL patients at diagnosis and 6 months later. Patients were classified as progressors or non-progressors.
After thawing, 1×107 cells were stained for the detection of surface and intracellular markers using an in-house developed 42-color full-spectrum cytometry panel. Samples were acquired on a 5-laser Cytek Aurora cytometer and analyzed with OMIQ software.
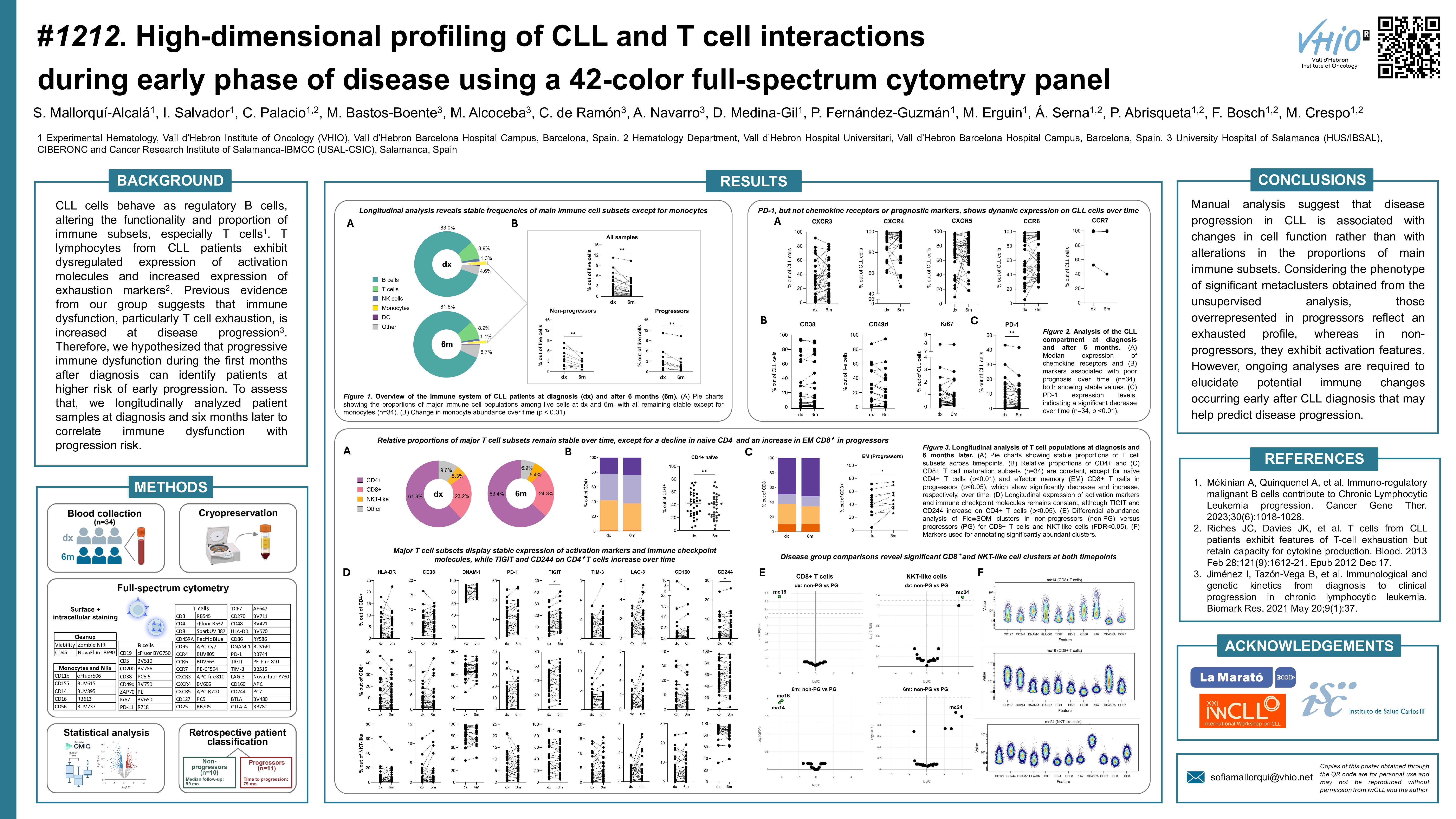
Results
After a median follow-up of 99 months, the median time to progression was 79 months for progressors. The proportion of main immune populations (B, T, and NK cells) remained stable at 6 months compared to diagnosis, except for a decrease in monocytes, regardless of patient progression (p=0.0069). Similarly, the expression of chemokine receptors (CXCR3, CXCR4, CXCR5, CCR6, CCR7) and poor prognosis markers (CD38, CD49d, Ki67, ZAP-70) in CLL cells remained constant. The frequency of naïve CD4+ T cells was significantly reduced at 6 months from diagnosis (p=0.0075). In addition, CD4+ T cells showed higher levels of TIGIT (p=0.0151) and CD244 (p=0.0163) over time. On the contrary, the proportion of CD8+ subsets and the expression of activation and exhaustion markers were unaltered throughout the evaluated period, as well as for NKT cells.
When comparing timepoints, an increased CD4:CD8 ratio was observed in non-progressors over time (p=0.0488), while it remained constant for progressors. CD4+ T cell subsets frequency and the expression of activation and exhaustion markers within the CD4+ compartment was also stable regardless of disease status. The proportion of CD8+ T cell subsets was unaltered in non-progressors, while in patients who progressed increased effector memory CD8+ T cells were observed (p=0.0137). Additionally, CD8+ T cells from non-progressors showed lower levels of CD244 at 6 months compared to diagnosis (p=0.0156). However, the expression of activation and exhaustion markers in the CD8+ population remained constant in progressors. The frequency of NKT cells and the expression of activation and exhaustion markers within this compartment did not fluctuate over time across patient groups.
To capture T-cell heterogeneity, we followed an unsupervised approach involving dimensionality reduction (UMAP), FlowSOM clustering, annotation, and statistical analysis of resulting clusters using edgeR for differential abundance testing. A total of 28 metaclusters (mc) were identified in CD4+, CD8+ and NKT cells. We observed stable cluster sizes over time within the aforementioned cell populations in all patient samples. At diagnosis, we detected an increased size of mc16 (FDR=0.0191) in CD8+ T cells from patients who did not progress compared to patients that progressed. This metacluster is enriched in terminally differentiated effector memory cells expressing CD127, CD244, CD49d and CD95, indicating a cytotoxic, partially exhausted but with a potentially active phenotype. Additionally, NKT cells from progressors showed an increased abundance of mc24 (FDR=0.0434) at diagnosis. In this case, mc24 is enriched in unconventional CD8+ terminally differentiated effector memory cells expressing DNAM-1, TIGIT, CD244, CD49d and CD95. This suggests chronic activation alongside functional exhaustion, as indicated by TIGIT and CD244 co-expression. Comparing samples obtained at 6 months, CD8+ T cells exhibited higher counts of mc14 (FDR=0.0136), and mc16 (FDR=0.0112) in non-progressors, as observed at diagnosis for mc16. The detected mc14 increase in size in patients that did not progress is characterized by the expression of Ki67, HLA-DR, DNAM-1, CD49d and CD95, indicating highly activated and potentially cytotoxic proliferative cells. Finally, no significant clusters were observed in NKT cells at 6 months.
Considering the phenotype of significant metaclusters, those overrepresented in progressors reflect an exhausted profile, whereas in non-progressors, they exhibit activation features. However, ongoing analyses are required to elucidate potential immune changes occurring early after CLL diagnosis that may help predict disease progression.
Keywords : tumor microenvironment, T-cell exhaustion, full-spectrum cytometry
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: Fundació La Marató de TV3