Authors
Lorinda Soma, Winston Lee, Olga V. Danilova, Jessica Hughes, Parastou Tizro, Matt Mei, Alexey V. Danilov, Joo Song.
Introduction
Measurable residual disease assessment (MRD) in chronic lymphocytic leukemia (CLL) has prognostic significance and has served as an endpoint in clinical trials. While not routinely used in clinical practice, MRD may guide therapy in the future. MRD can be quantified by flow cytometry (FC) and by next-generation sequencing, with each method having its own pitfalls. With additional treatments being introduced in CLL, we aimed to develop a FC-based MRD assay that could quantify CLL in the setting of both anti-CD19 and anti-CD20 therapies. As part of the development, we included samples from patients treated with anti-CD19 antibody tafasitamab on a clinical trial (combined with zanabrutinib; NCT05718869)
Methods
We analyzed 61 samples in two cohorts:
1. Development cohort:
a. 31 CLL cases (29 peripheral blood and 2 bone marrow)
i. 13 patients on active targeted antibody therapy: 11 on tafasitamab (anti-CD19), 1 on obinutuzimab and 1 on rituximab.
ii. 9 patients on Bruton Tyrosine Kinase (BTK) inhibitor or BCL2 inhibitor therapy.
iii. 8 patients on surveillance.
b. 10 normal peripheral bloods
2. Implementation cohort: 20 CLL cases (16 peripheral blood and 4 bone marrow)
a. 3 patients on tafasitamab
b. 13 patients on BTK inhibitor and/or BCL2 inhibitor
c. 4 patients on surveillance (1 received liso cel CART 4 months prior)
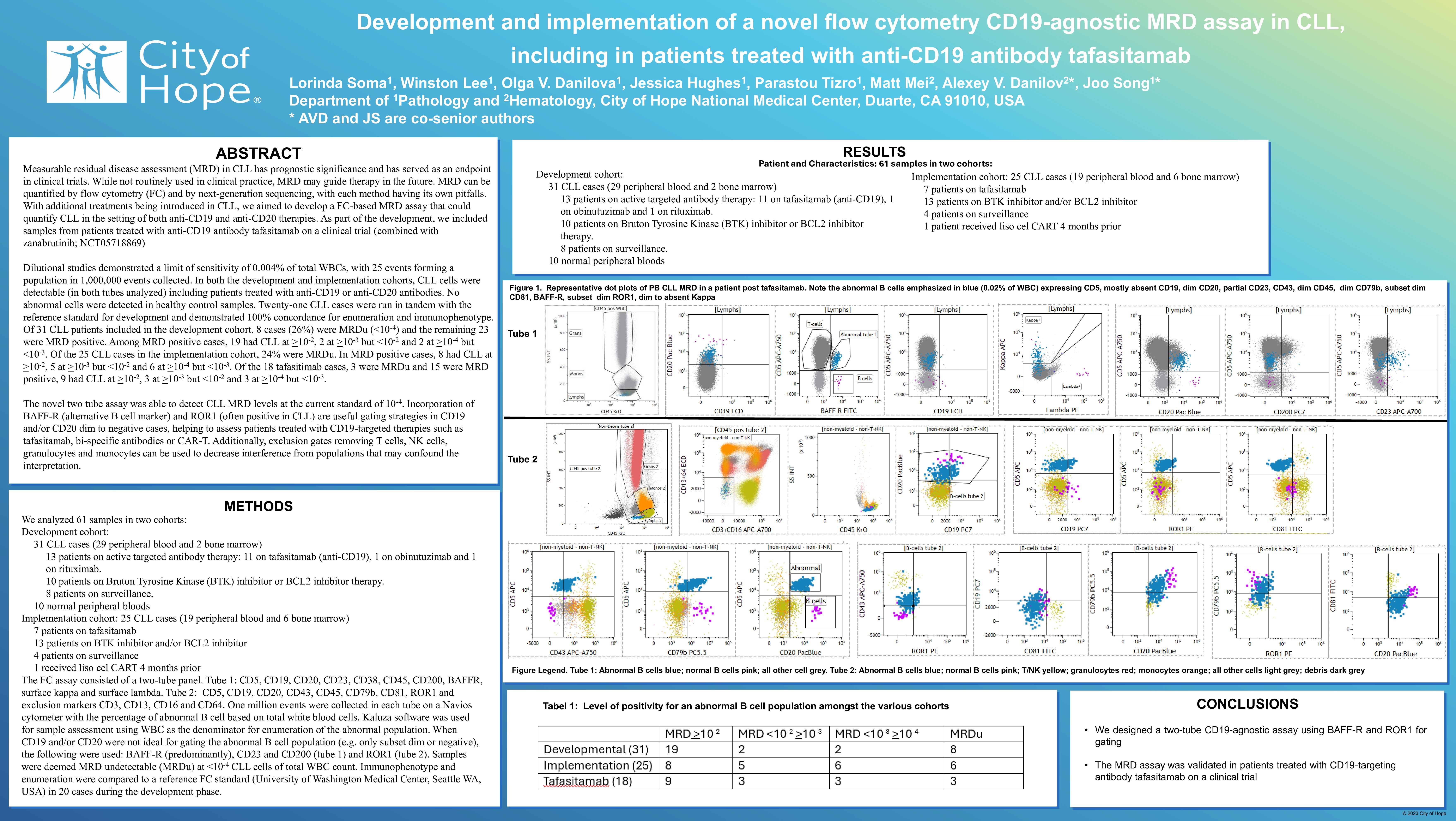
The FC assay consisted of a two-tube panel. Tube 1: CD5, CD19, CD20, CD23, CD38, CD45, CD200, BAFFR, surface kappa and surface lambda. Tube 2: CD5, CD19, CD20, CD43, CD45, CD79b, CD81, ROR1 and exclusion markers CD3, CD13, CD16 and CD64. One million events were collected in each tube on a Navios cytometer with the percentage of abnormal B cell based on total white blood cells. Kaluza software was used for sample assessment using WBC as the denominator for enumeration of the abnormal population. When CD19 and/or CD20 were not ideal for gating the abnormal B cell population (e.g. only subset dim or negative), the following were used: BAFF-R (predominantly), CD23 and CD200 (tube 1) and ROR1 (tube 2). Samples were deemed MRD undetectable (MRDu) at < 10-4 CLL cells of total WBC count. Immunophenotype and enumeration were compared to a reference FC standard (University of Washington Medical Center, Seattle WA, USA) in 20 cases during the development phase.
Results
Dilutional studies demonstrated a limit of sensitivity of 0.004% of total WBCs, with 25 events forming a population in 1,000,000 events collected. In both the development and implementation cohorts, CLL cells were detectable (in both tubes analyzed) including patients treated with anti-CD19 or anti-CD20 antibodies. No abnormal cells were detected in healthy control samples. Twenty CLL cases were run in tandem with the reference standard for development and demonstrated 100% concordance for enumeration and immunophenotype. Of 31 CLL patients included in the development cohort, 8 cases (26%) were MRDu ( < 10-4) and the remaining 23 were MRD positive. Among MRD positive cases, 19 had CLL at >10-2, 2 at >10-3 but < 10-2 and 2 at >10-4 but < 10-3. Of the 20 CLL cases in the implementation cohort, 25% were MRDu. In MRD positive cases, 8 had CLL at >10-2, 4 at >10-3 but < 10-2 and 4 at >10-4 but < 10-3. Of the 14 tafasitimab cases, 1 was MRDu and 13 were MRD positive, 9 had CLL at >10-2, 3 at >10-3 but < 10-2 and 1 at >10-4 but < 10-3.
Discussion
The novel two tube assay was able to detect CLL MRD levels at the current standard of 10-4. Incorporation of BAFF-R (alternative B cell marker) and ROR1 (often positive in CLL) are useful gating strategies in CD19 and/or CD20 dim to negative cases, helping to assess patients treated with CD19-targeted therapies such as tafasitamab, bi-specific antibodies or CAR-T. Additionally, exclusion gates removing T cells, NK cells, granulocytes and monocytes can be used to decrease interference from populations that may confound the interpretation.
* AVD and JS are co-senior authors
Keywords : MRD, tafasitamab, CD19
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: