Authors
Parry HM, Cook J, Peckham N, Phillips N, Talbot G, Abhishek, A Duley L, Hodges M, Roberts T, Barber V, Francis A, Shields A, Hoogeboom R, Willett B, Scott S, Parry-Jones N, Eyre T, Hutchinson C, Wandroo F, Paneesha S, Murray D, Jenkins S, Moss P, Heartin E, Martinez-Calle N, Patten P.
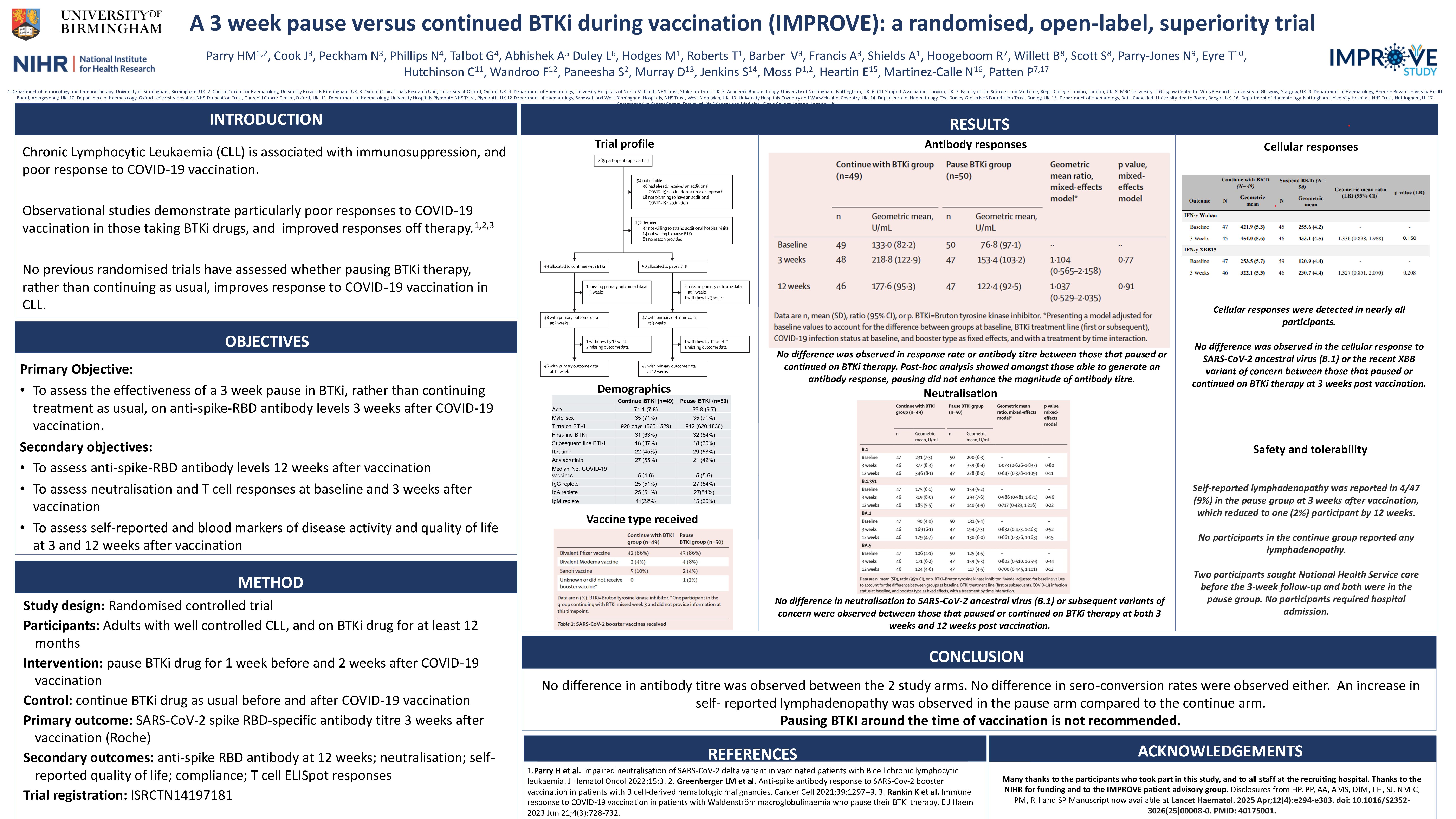
CLL is the commonest leukaemia and associated with profound immunosuppression. Bruton Tyrosine Kinase inhibitors (BTKi) have revolutionised CLL management, however therapy impairs vaccine-induced immunity and serious infection is observed in around 20%. Pausing therapy for a short duration has been postulated to improve vaccine immunity but has never been tested in a randomised setting. We evaluated if a three-week pause of BTKi treatment improved spike protein (S) receptor binding domain (RBD) immunity to SARS-CoV-2 vaccination whilst maintaining disease control.
We performed an open-label, 2-arm parallel group, randomised trial in secondary-care haematology clinics in 11 UK hospitals. Patients aged 18 and over, diagnosed with CLL and currently taking BTKi therapy for at least 12 months were eligible. Participants were randomised (1:1 stratified by BTKi therapy line) to pause BTKi for 3 weeks, starting 6 days before their COVID-19 vaccine booster date or continue therapy as usual. The primary outcome measure was anti-spike-RBD-specific antibody titre 3 weeks post-vaccination. Secondary outcomes included antibody titre at 12 weeks, neutralisation to variants of concern and cellular responses measured by IFNγ. Trial registration: ISRCTN 14197181.
Between 10/10/2022 and 08/06/2023, 99 individuals were randomised to pause (n=50) or continue (n=49) their BTKi therapy. Median prior doses of COVID-19 vaccinations at enrolment was 5. At three-weeks post vaccine, the geometric mean (SD) RBD antibody level was 218.8 (122.9) and 153.4 (103.2) U/ml in the continue and pause arms respectively with geometric mean ratio (95%CI) 1.104 (0.565-2.158), p=0.772, mixed-effects model and no difference in seroconversion between the 2 arms observed. Therapy interruption did not improve antibody neutralisation or cellular IFNγ response to ancestral B.1 or variants of concern (VOCs) at 3 weeks post vaccine either. No intervention related serious adverse events were reported although self reported lymphadenopathy was more common in the pause arm than those that continued on therapy.
Further supportive scientific work studying the heterogeneous responses observed in either arm, is now in progress. This study provides no evidence that pausing BTKi around the time of vaccination is beneficial for immunity and should not be recommended in clinical practice.
Funding NIHR:151892.
Keywords : Vaccination, BTKi, antibody
Please indicate how this research was funded.: NIHR EME trial
Please indicate the name of the funding organization. : NIHR