Authors
Aiysha Khan BSc, Marie Hodges PhD, Jared Darcy MSc, Paul Moss PhD, Becky Sinclair MSc, Thomas Roberts MSc, Piers Patten MBChB PhD, Jianmin Zuo PhD, Helen Parry MBChB PhD.
Background
Humoral immunity to vaccination is known to be impaired on continuous BTKi therapy. In comparison, less is known regarding cellular responses to vaccination. Cellular immunity has been reported to improve during covalent BTKi therapy, evidenced through enhanced antigen specific responses to latent herpes viruses and the improved generation of CAR-T cells amongst patients previously treated with BTKi. Several mechanisms have been postulated to underpin this improvement. An appreciation of the contribution of NK and T cell antigen-specific immunity and a comparison of functional responses elicited by the different covalent BTKi drugs remains unknown.
Aims
-To assess and compare antigen-specific cellular responses amongst patients taking either ibrutinib or acalabrutinib 3 weeks following vaccination and compare with humoral responses.
-To investigate the relative contribution of NK and T cell responses to 2 different pools of overlapping peptides from SARS-CoV-2 spike glycoprotein (S1 and S2) following vaccination.
-To assess the phenotypic and transcriptional differences between ‘strong’ and ‘poor’ cellular responders.
– To identify whether NK cells can respond to bioinformatically predicted individual peptides independent of T cell stimulation.
Methods
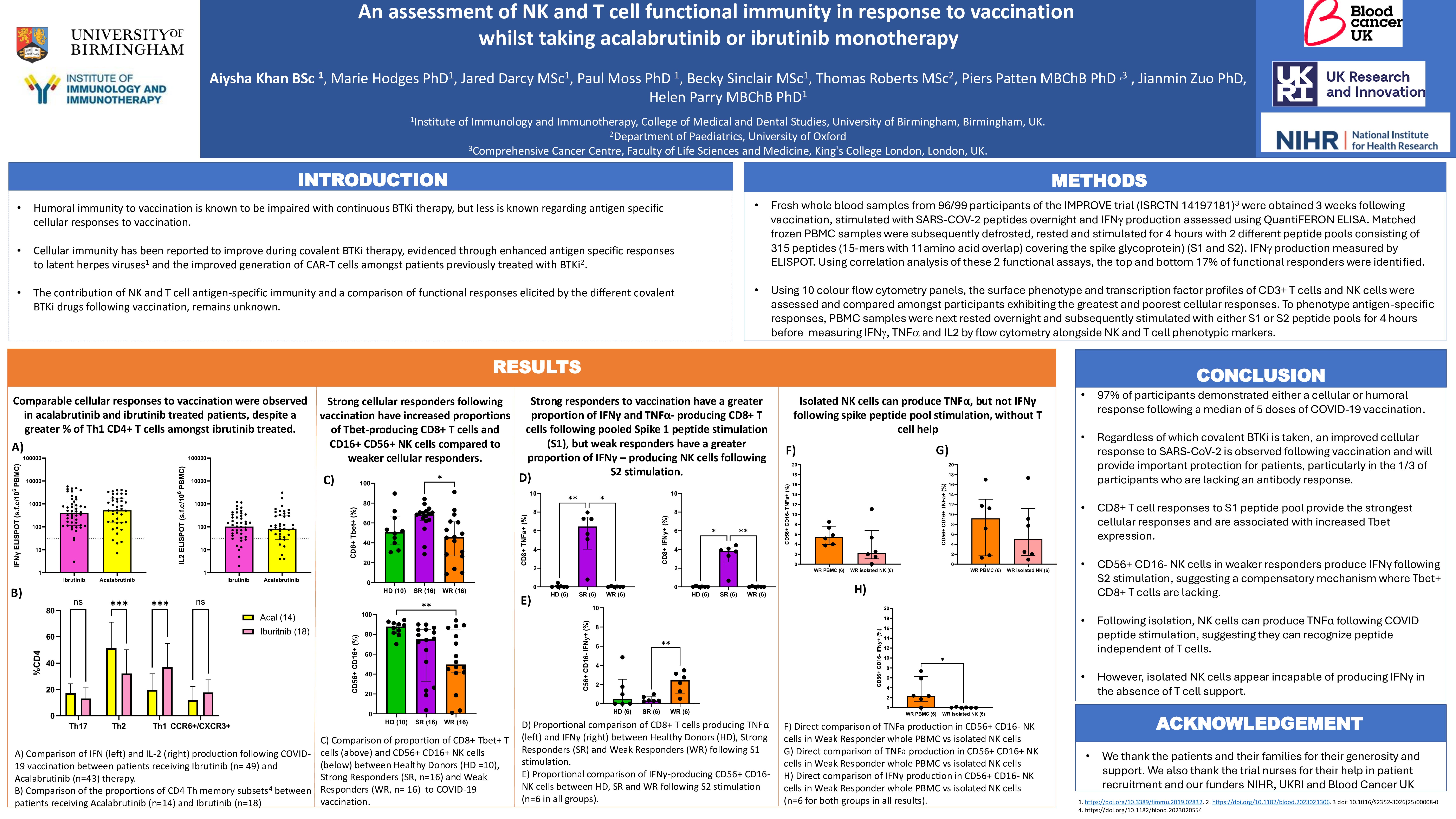
Fresh whole blood samples from participants of the IMPROVE trial (ISRCTN 14197181) were obtained 3 weeks following vaccination, stimulated with SARS-COV-2 peptides overnight and IFN production assessed using QuantiFERON ELISA (Qiagen 626715). Matched frozen PBMC samples were subsequently defrosted, rested and stimulated for 4 hours with 2 different peptide pools consisting of 315 peptides (15 mers with 11aa overlap) covering the spike glycoprotein (JPT peptides) and IFN production measured by ELISPOT. Using correlation analysis of these 2 functional assays, the top and bottom 17% of functional responders were identified.
Using 10 colour flow cytometry panels, the surface phenotype and transcription factor profiles of CD3+ T cells and NK cells were assessed and compared amongst participants exhibiting the greatest and poorest cellular responses.
To phenotype antigen-specific responses, PBMC samples were next rested overnight and subsequently stimulated with either S1 or S2 peptide pools for 4 hours before measuring IFN, TNF and IL2 by flow cytometry alongside NK and T cell phenotypic markers.
Results
Functional T cell responses were observed in 93% of participants (86/92) after a median of 5 doses of SARS-CoV-2 vaccination. Only 2 participants (2%) lacked either an antibody or cellular response to vaccination. Cellular responses were increased following vaccination (p=0.0005) compared to pre-vaccine samples, with no difference observed between those taking acalabrutinib (n=43) and ibrutinib (n=49) therapy. Despite this, amongst ibrutinib treated patients, a higher proportion of Th1 CD4+ T cells were observed compared to those taking acalabrutinib (ibrutinib 34.5% vs 16.1%; p=0.0007). The reverse was noted, with higher proportions of Th2 CD4+ T cells observed amongst acalabrutinib patients.
Fresh and frozen correlative analysis of Spike peptide responses showed a good correlation r=0.589 p= < 0.0001. A comparison of the best and worst cellular responders found no difference in the percentage of CD3+, CD4+ or CD8+ T cells. Amongst CD8+ T cells, expression of the transcription factor Tbet, was significantly higher in the best responders (median 68.53%) compared to those with poorer cellular responses (median 45.65%) (p=0.0045). No difference in NK % or phenotypic expression of CD16+/ CD56+ was noted between the best and poorest responders.
Amongst best cellular responders, the dominant peptide response was observed amongst CD8+ T cells (rather than CD4+ T or NK cells) and was predominantly combined IFN and TNF production in response to the RBD (receptor binding domain) containing S1 peptide pool. In contrast, amongst poorer responders, NK responses dominated compared to CD4 or CD8+ T cell responses, with TNF secretion observed in response to the S2 peptide pool. Work is now ongoing to establish if NK responses observed are dependent on T cell stimulation or peptide specific and results will be presented at IWCLL.
Conclusion
In conclusion, regardless of which covalent BTKi is taken, an improved cellular response to SARS-CoV-2 is observed following vaccination and will provide important protection for patients, particularly where antibody response are lacking. CD8+ T cell responses to S1 peptide pool provide the strongest cellular responses and are associated with increased Tbet expression. In comparison NK responses may compensate for some patients with poorer CD3+ T cell responses and work is now ongoing to identify if this is T cell independent.
Keywords : cellular, immunity, BTKi
Please indicate how this research was funded. : Laboratory research grant and trial funding.
Please indicate the name of the funding organization.: NIHR for trial and sample collection. MRC/BCUK for the laboratory work.