BRD4 maintains the surface phenotype of Chronic Lymphocytic Leukemia cells (438kB pdf)
Authors
Athina Georgiou, Daniel Friedman, Ka Ka Yu, Drshika P Mehtani, Piers EM Patten, Chi Wai Eric So, Robbert Hoogeboom.
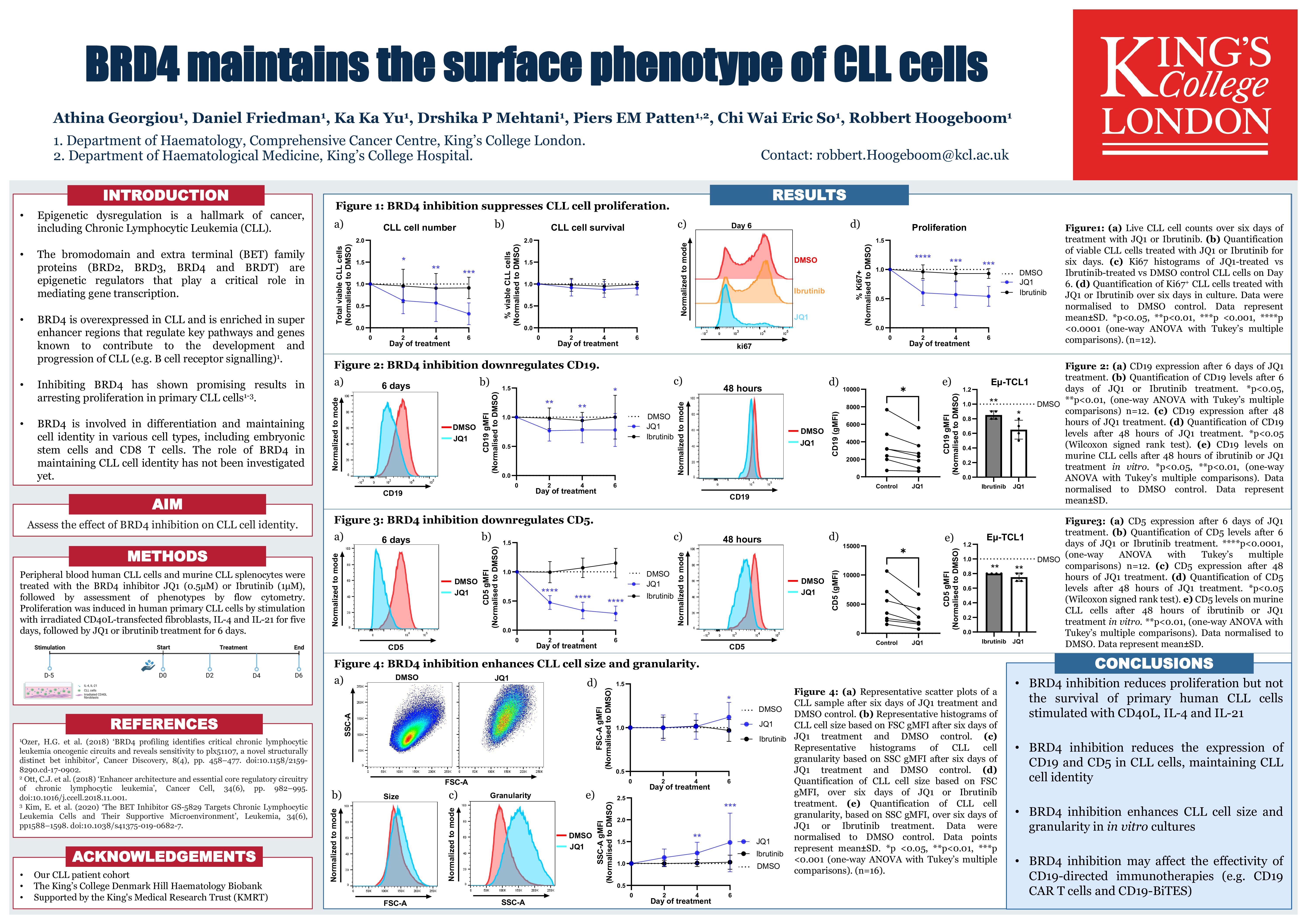
Global changes in the epigenetic landscape are a hallmark of cancer, including chronic lymphocytic leukemia (CLL). The epigenetic regulator Bromo domain-containing protein 4 (BRD4) is overexpressed in CLL and controls super enhancer regions that regulate the expression of multiple genes and pathways involved in the development and progression of CLL (e.g. B-cell receptor signalling), emphasizing the importance of BRD4 in CLL pathology (Ozer et al, Cancer Discov. 2018). In other cell types, BRD4 has also been implicated in differentiation and maintaining cell identity. However, the effect of BRD4 inhibition on the differentiation of CLL cells has not been explored yet.
To study the effect of BRD4 inhibition on differentiation of CLL cells, we exposed CLL cells to the BRD4 inhibitor JQ1 and analyzed the surface phenotype by flow cytometry. In the CLL cell lines MEC-1 and PCL-12, we observed a 47% and 60% reduction CD19 expression after 72 hours of 1 µM JQ1, respectively. In murine CLL cells derived from Eu-TCL1-transgenic mice (n=4), 48 hours of BRD4 inhibition with 0.5 µM JQ1 in vitro reduced CD19 expression by 35% and in primary human CLL cells (n=7), 48 hours of 0.5 µM JQ1 treatment in vitro reduced CD19 expression by 25%. In addition, we found CD5 expression reduced by 40% in human primary CLL cells (n=7) and 25% in murine primary CLL cells (n=4), demonstrating that BRD4 maintains the phenotype of CLL cells. Of note, CD5 levels on T cells remained stable, indicating that this effect is specific for B cells.
In vivo, CLL cells maintain an activated memory B cell phenotype with limited differentiation into plasma cells. To investigate the effect of BRD4 inhibition on terminal differentiation of CLL cells, we cultured primary CLL cells on CD40L-transfected fibroblasts, in the presence of IL-4 and IL-21, conditions that promote terminal differentiation of healthy donor B cells but not primary CLL cells. After five days of stimulation, when CLL cells are proliferating, the CLL cells were treated with JQ1 (0.5 µM) or the BTK inhibitor Ibrutinib (1 µM) for a further six days. As expected, treatment with JQ1 had no effect on the viability of CLL cells, whilst proliferation of CLL cells was significantly suppressed in all patients tested (n=16). In contrast, ibrutinib, a standard-of-care first-line treatment for CLL, had a minimal effect on proliferation in these culture conditions. Interestingly, BRD4 inhibition, but not BTK inhibition, induced a 10% increase in cell size and a 50% increase in granularity as determined by flow cytometry and strikingly reduced the surface levels of CD5 and CD19. Increased size and granularity and reduced CD19 expression could be indicative of terminal differentiation into plasma cells. To investigate potential differentiation into plasma cells, we analysed surface CD138 expression by flow cytometry and found a 2-fold increase in JQ1-treated CLL cells but not DMSO or ibrutinib treated cells (n=12). In addition, May-Grunewald Giemsa staining revealed eccentric nuclei and conspicuous perinuclear hofs, features of plasmacytic differentiation, in BRD4 inhibited cells.
In conclusion, our findings demonstrate that inhibition of BRD4 modulates the phenotype of CLL cells by reducing CD19 and CD5 and inducing features of plasma cells, opening new avenues to develop differentiation therapy for CLL and other B cell malignancies.
Keywords : Leukemia, differentiation, phenotype
Please indicate how this research was funded. : KMRT studentship 2020
Blood Cancer UK – Project grant 22006
Please indicate the name of the funding organization.: King’s Medical Research Trust (KMRT) Blood Cancer UK (BCUK)