Authors
Catherine Cho, Robert Redd, J. Erika Haydu, P. Connor Johnson, Jeffrey Barnes, Ronald Takvorian, Ephraim Hochberg, Josie Ford, Jeremy Abramson, Jacob D. Soumerai.
Introduction
Secondary central nervous system (CNS) involvement is a rare occurrence in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Our understanding of this rare complication, and how to approach treatment for these patients, is largely drawn from case reports. Herein, we evaluated the incidence of CNS involvement in CLL/SLL, and we evaluated real-world evidence regarding treatment for this rare complication.
Methods
This retrospective observational study used an IRB-approved lymphoid malignancy registry. Eligible patients had CLL/SLL and were cared for at Massachusetts General Hospital (MGH). Patients were identified through retrospective chart review of CLL/SLL patients in the lymphoid malignancy registry. Cumulative incidence of CNS involvement was calculated from CLL/SLL diagnosis to CNS diagnosis and estimated using the Kaplan-Meier method (censored when last known alive without CNS involvement). CNS response was defined as resolution of associated CNS disease symptoms for at least 3 months and, as applicable, reduction/resolution by CSF cytology and/or MRI. Analyses were performed using R version 4.4.2.
Results
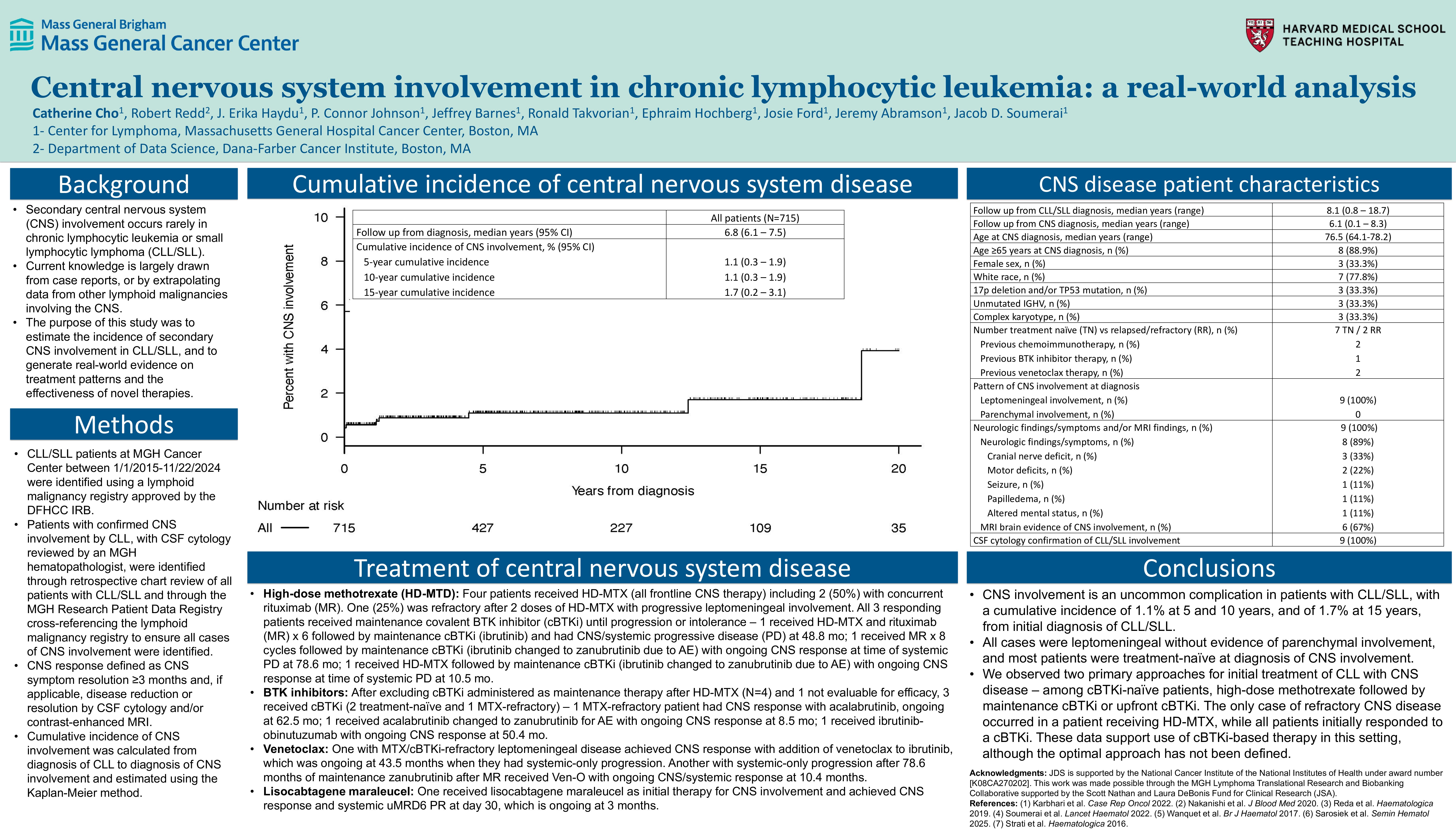
Between 1/1/15-11/22/24, among 715 consecutive CLL/SLL patients, with a median follow-up of 6.8 years, the cumulative incidence of CNS involvement was 1.1% (95% CI 0.3-1.9) at 5 years and 1.7% (95% CI 0.2-3.1) at 15 years. Of 9 (1.3%) patients with CNS disease, the median time from initial diagnosis of CLL/SLL to diagnosis of CNS involvement was 13.8 months (range, 0-224.1), all 9 patients had cerebrospinal fluid (CSF) cytology reviewed by an MGH hematopathologist confirming increased number of clonal B cells in the CSF without significant red cell contamination and diagnostic of leptomeningeal involvement by CLL/SLL, and all 9 patients had neurologic findings (n=8; cranial nerve deficits [n=3], motor deficits [n=2], seizure [n=1], papilledema [n=1], and altered mental status [n=1]) and/or MRI evidence of leptomeningeal involvement (n=6). The median age was 76.5 years (range, 64.1-78.2), the median absolute lymphocyte count was 10,200/𝜇L (range, 220-126,630/𝜇L), 33.3% (n=3) had TP53 mutation or 17p deletion, 44.4% (n=4) had unmutated IGHV, and 33.3% (n=3) had 3 or more karyotypic abnormalities. The median number of therapies prior to secondary CNS diagnosis was 0 (range, 0-5), including 7 treatment-naïve and 2 previously treated. One of the patients had systemic Richter’s transformation (diffuse large B-cell lymphoma) but only CLL/SLL was demonstrated in the CNS with exhaustive CNS evaluation (this patient was included as a case of CNS disease but excluded from treatment results below).
The median number of therapies for CNS involvement was 2 (range, 1-4) and the median follow-up calculated from CNS diagnosis was 6.1 years (range, 0.1-8.3). Among 7 cBTKi-naïve patients, 4 received HD-MTX: 1 MTX-refractory in an ongoing response after 62.5 mo on cBTKi (acalabrutinib); 3 responded to HD-MTX followed by maintenance cBTKi (2 systemic-only PD at 78.6 [zanubrutinib] and 10.5 mo [zanubrutinib]; 1 CNS/systemic PD at 48.8 mo on cBTKi [ibrutinib]); 3 received upfront cBTKi: 2 responded (ongoing at 50.4 [ibrutinib] and 8.5 mo [zanubrutinib]); 1 was not evaluable for efficacy evaluation (discontinued cBTKi [acalabrutinib] within 2 weeks due to concurrent candidemia and septic shock, and opted against further therapy). One additional patient had CLL refractory to cBTKi and venetoclax at the time of CNS diagnosis, and this patient received lisocabtagene maraleucel with uMRD6 PR at 1 mo, with response ongoing at 3 mo. An additional patient with MTX/cBTKi-refractory leptomeningeal disease achieved durable CNS response with venetoclax, which was ongoing at 43.5 mo, at which point they had systemic-only PD and initiated pirtobrutinib with ongoing CNS/systemic response at 5.2 mo on pirtobrutinib.
Conclusion
The cumulative incidence of secondary CNS involvement in patients with CLL/SLL cared for at an academic cancer center was 1.1% at 5 and 10 years, and 1.7% at 15 years from diagnosis, respectively. We observed two approaches for initial treatment of cBTKi-naïve patients with CLL/SLL with CNS disease – (1) upfront cBTKi or (2) HD-MTX followed by maintenance cBTKi. While the optimal approach has not been defined, upfront use of a cBTKi achieved rapid and durable CNS responses, and cBTKi monotherapy is generally our preferred approach in this setting. We also observed CNS responses in patients receiving venetoclax or lisocabtagene maraleucel, suggesting a role for these agents in cBTKi-refractory or intolerant patients.
Keywords : chronic lymphocytic leukemia, central nervous system, leptomeningeal
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: MGH Lymphoma Translational Research and Biobanking Collaborative supported by the Scott Nathan and Laura DeBonis Fund for Clinical Research