Authors
Maya Koren-Michowitz, Mariia Mikhaleva, Thomas Chatzikonstantinou, Christian Brieghel, Christina Papaggelou, Eva Minga, Celso Arrais, Sotiria Besikli-Dimou, Amalia Cerutti, Michael Doubek, Lisbeth Enggaard, Blanca Espinet, Blanca Ferrer-Lores, Jose Antonio Garcia Vela, Massimo Gentile, Eva Gimeno Vázquez, Odit Gutwein, Yair Herishanu, Paula Jablonowska, Ozren Jaksic, Par Josefsson, Elzbieta Kalicińska, Maria Kislova, Enrico Lista, Ioannis Kotsianidis, Juan Marquet, Enrica Antonia Martino, Ciaran McAuley, Maria José Mela Osorio, Riccardo Moia, Claudia Mosquera, Eugene Nikitin, Miguel Arturo Pavlovsky, Verena Pfister, Merve Savas, Lydia Scarfò, Matjaz Sever, Lev Shvidel, Martin Simkovic, Niki Stavroyianni, Persefoni Talimtzi, Antigoni Tranidou, Andrea Visentin, George Vrachiolias, Ewa Wasik-Szczepanek, Tomasz Wróbel, Munci Yagcı, Alessandro Cellini, Anastasia Chatzidimitriou, Gianluca Gaidano,Mary Ann Anderson, Matthew S. Davids, Jennifer R. Brown, Carsten U. Niemann, Kostas Stamatopoulos, Paolo Ghia, InhyeE. Ahn.
Background
CNS involvement (CNSi) of chronic lymphocytic leukemia (CLL) is a rare condition with no consensus on diagnosis and limited evidence for the management and outcome.
Methods
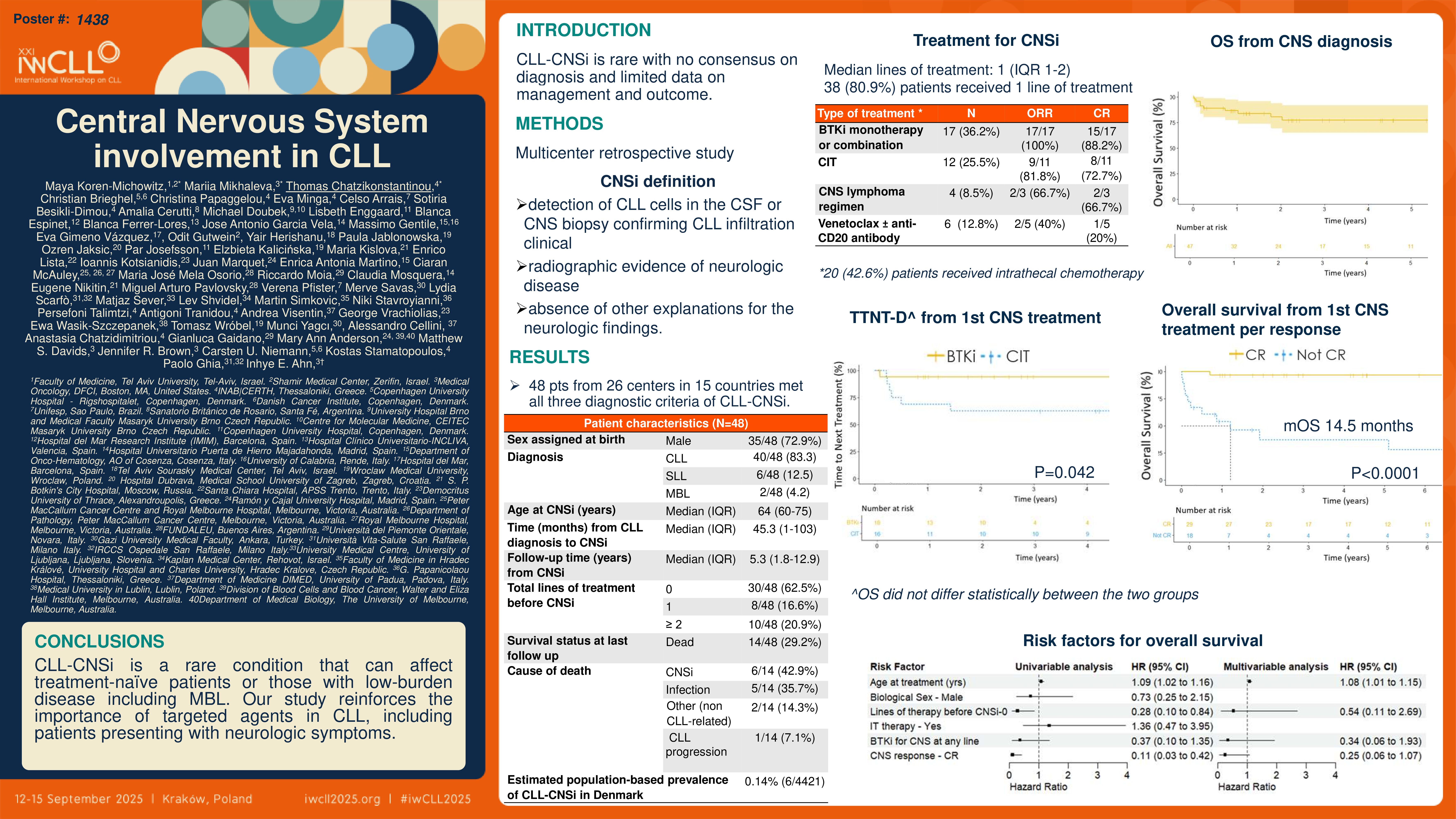
This is an international, multicenter, retrospective study conducted by the European Research Initiative on CLL (ERIC). The study defined CNSi of CLL by: 1) detection of CLL cells in the cerebrospinal fluid (CSF) or confirmation of CLL infiltration of the CNS based on a tissue biopsy, 2) clinical or radiographic evidence of neurologic disease, and 3) the absence of other explanations for the neurologic findings. We defined complete remission (CR) of CNS disease by resolution of clinical symptoms with normalization of CSF and neuro-imaging findings. Partial response (PR) was defined by improvement of clinical symptoms with normalization of CSF and at least a 50% decrease of involved sites in neuro-imaging. For the CLL-CNSi prevalence calculation, we restricted the analysis to data from the Danish Lymphoid Cancer Research (DALY-CARE) data resource.
Results
A total of 48 patients from 26 centers in 15 countries met all three diagnostic criteria of CLL-CNSi. Median age at diagnosis of CNSi was 64 years. Most patients were males (73%), had Binet stage A at CLL diagnosis (61%), and had untreated CLL at the time of CNSi (63%). Motor impairment was the most common symptom (38%) followed by visual impairment (32%).
Overall, 4421 patients in DALY-CARE had medical notes from hematologists in the electronic health records covering half of the Danish population. Seven patients were treated for CLL-CNSi, while 6 of them met the pre-defined diagnostic criteria for CLL-CNSi. Thus, the estimated population-based prevalence of CLL-CNSi in Denmark was 0.14% (6/4421).
Of 48 patients in our cohort, 47 received CNSi-directed treatment. Over 80% of patients received a single line of CNSi-directed treatment (38/47, 80.9%). Smaller proportions of patients received two (6/47, 12.7%) to three or more lines of treatment (3/47, 6.4%). Intrathecal chemotherapy was administered in 20/47 (42.6%) patients, and 3/47 (6.4%) proceeded to allogeneic stem cell transplantation. In the first line setting, Bruton tyrosine kinase inhibitors (BTKis) given as monotherapy or in combination with an anti-CD20 antibody, were most commonly used (18/47, 38.3%), followed by fludarabine, cyclophosphamide, and rituximab (FCR; 7/47, 14.9%), venetoclax plus anti-CD20 antibody (4/47, 8.5%), and other chemoimmunotherapy (CIT) regimens typically used in CNS lymphoma (4/47, 8.5%). In the second-line setting, BTKi-based treatment with ibrutinib (3/9, 33.3%) and CNS lymphoma regimens (3/9, 33.3%) were most commonly used.
Rates of overall response (ORR) and CR for the CNS disease to any CNSi-directed treatment were 82.9% (34/41) and 70.7% (29/41), respectively. Patients treated with BTKis (monotherapy or in combination with other treatment modalities) had the highest ORR (17/17, 100%) and CR (15/17, 88.2%), followed by CIT (ORR: 9/11, 81.8%; CRR: 8/11, 72.7%) and CNS lymphoma protocols (ORR: 2/3, 66.7%; CRR: 2/3, 66.7%).
Patients who achieved a CR had significantly longer TTNT-D and OS (median not reached for both TTNT-D and OS) than those without a CR (median TTNT-D 3.8 months, median OS 14.5 months). Patients who received CNSi-directed treatment as their first-ever treatment for CLL (treatment-naïve CNSi) had a more favorable outcome than those who had been previously treated for CLL and presented with CNSi (relapsed/refractory CNSi). Median TTNT-D and OS were not reached for the treatment-naïve CNSi, and were 23 and 100 months, respectively, for the relapsed/refractory CNSi. Finally, patients treated with BTKis for CLL-CNSi had a longer TTNT-D compared with those treated with CIT including CNS lymphoma regimens (5-year TTNT-D 94.1% [95% CI=83.6%-100%] vs. 64.2% [95% CI=28.7%-100%]); OS did not differ statistically between the two groups.
We next performed a univariable analysis to identify risk factors associated with inferior OS in patients with confirmed CNSi. Older age, not achieving CR after the first CNS-directed treatment, and the number of treatment lines for CLL before CNS-directed treatments were associated with worse OS in the univariable analysis. In the multivariable analysis, age and CR status after the first CNS-directed treatment correlated with OS, although only age reached statistical significance.
Conclusions
CLL-CNSi is a rare condition that can affect treatment-naïve patients or those with low-burden disease including MBL. Our study reinforces the importance of targeted agents in CLL, including patients presenting with neurologic symptoms.
Keywords : CLL-CNSi, BTKi
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: