Authors
Talha Munir, Keri Yang, Sheng Xu, Rhys Williams, Mazyar Shadman
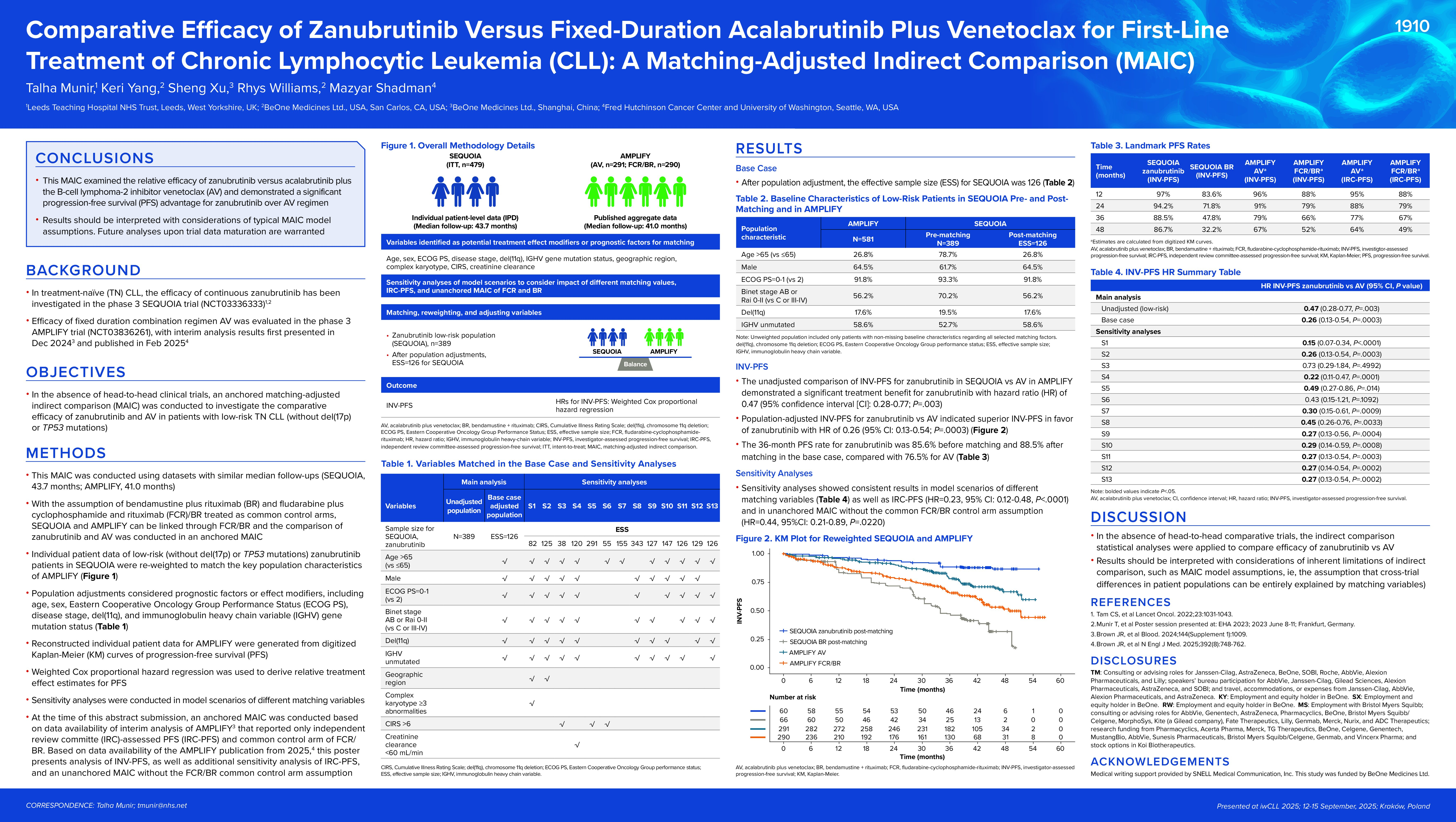
Background
In treatment-naive (TN) CLL, the efficacy of continuous ZANU has been investigated in the phase 3 SEQUOIA trial (NCT03336333) and efficacy of fixed duration combination regimen AV has been reported in the interim analysis of phase 3 AMPLIFY trial (NCT03836261). In the absence of head-to-head clinical trials, an anchored MAIC was conducted to investigate the comparative efficacy of ZANU and AV in low-risk TN CLL patients (without del(17p) or TP53 mutation).
Methods
This MAIC was conducted using datasets with similar median follow-ups (SEQUOIA, 40.52 months; AMPLIFY, 41 months). With the assumption of bendamustine plus rituximab (BR) and fludarabine plus cyclophosphamide and rituximab (FCR)/BR treated as common control arms, SEQUOIA and AMPLIFY can be linked through FCR/BR and the comparison of ZANU and AV were conducted in an anchored MAIC. Specifically, individual patient data of low-risk ZANU patients in SEQUOIA were re-weighted to match the key population characteristics of AMPLIFY and adjusted for age, sex, ECOG PS, disease stage, del(11q) and IGHV mutation status. Reconstructed individual patient data for AMPLIFY were generated from digitized Kaplan-Meier (KM) curves of progression-free survival (PFS). Sensitivity analyses were conducted in unanchored MAIC without the common control arm assumption, as well as in model scenarios of different matching variables.
Results
Relative PFS for ZANU vs AV in the unadjusted population were 0.42 (95% CI: 0.25-0.68; P<.0001). After matching adjustment, the effective sample size was 125.6 for SEQUOIA. PFS was superior for ZANU (HR for PFS = 0.23 [95% CI: 0.12-0.48]; P<.0001). The 36-month PFS rate for ZANU was 85.6% before matching and 88.5% after matching, compared with 76.5% for AV. Sensitivity analyses confirmed consistent results.
Conclusions
This MAIC examined the relative efficacy of ZANU versus AV and suggested a significant PFS advantage for ZANU over AV regimen. Results should be interpreted with considerations of MAIC model assumptions. Future analyses upon trial data maturation are warranted.
Keywords : CLL, zanubrutinib, BTKi
Please indicate how this research was funded.: BeOne Medicines Ltd.
Please indicate the name of the funding organization. : BeOne Medicines Ltd.