Authors
O.G Burling, B.J Kuss, S.M Hickey, O.G Best, L.A Thurgood.
Background
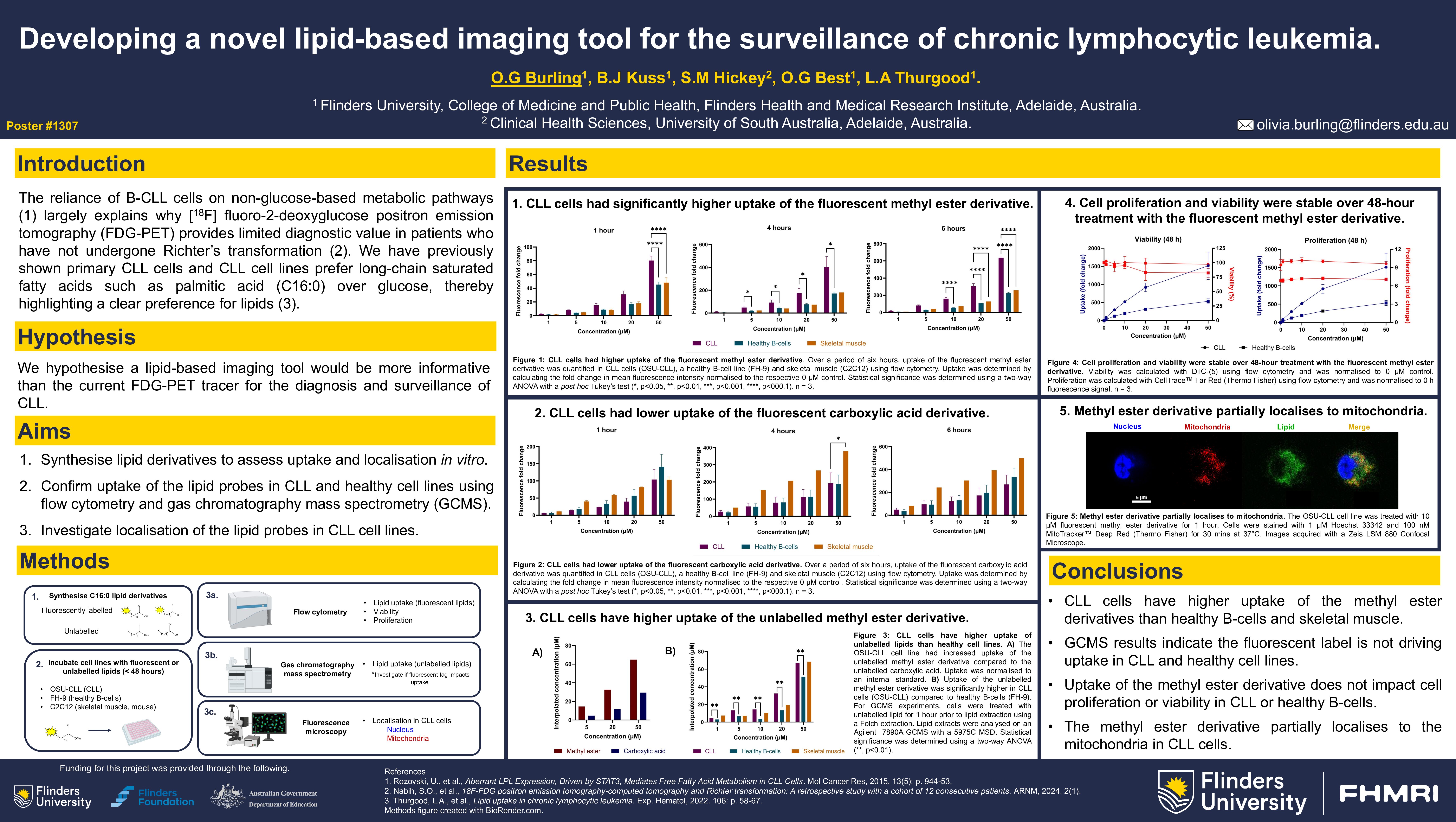
The reliance of B-lymphocytes on non-glucose-based metabolic pathways in chronic lymphocytic leukemia (Rozovski et al., Mol Cancer Res. 2016) largely explains why [18F] fluoro-2-deoxyglucose positron emission tomography (FDG-PET) provides limited diagnostic value in patients who have not undergone Richter’s transformation (Nabih et al., ARNM. 2024). We have previously shown that B-CLL cells preferentially sequester large amounts of palmitic acid (C16:0) over glucose and actively downregulate pathways involved in glucose uptake and utilisation, thereby highlighting a clear metabolic preference for lipids. We hypothesise a lipid-based imaging tool would be more informative for CLL diagnosis and surveillance than the current FDG-PET tracer. Four derivatives of palmitic acid were synthesised and evaluated in vitro to investigate the efficacy of a novel lipid-based imaging tool in CLL.
Methods
Four derivatives of palmitic acid were prepared following multi-step syntheses, including two fluorescently tagged and two fluorinated lipids as methyl ester and carboxylic acid derivatives. Uptake of the fluorescent lipids was quantified using flow cytometry in CLL (OSU-CLL, HG-3) and healthy (FH-9) B-lymphocyte lines and other lipid-avid tissue including murine skeletal muscle (C2C12). Cell lines were treated with up to 50 µM of each probe for < 48 hours and uptake was quantified by measuring mean fluorescence intensity. Cell proliferation was assessed concurrently by staining with CellTrace Far Red™ prior to incubation with the lipid. Viability at 50 µM after 48 hours was assessed using DiIC1(5). Uptake of the fluorinated lipids was assessed by incubating the cell lines listed above with up to 50 µM of lipid for 24 hours. Lipid extracts were isolated using Folch extraction and were derivatised prior to analysis using gas chromatography mass spectrometry (GCMS).
Results
Flow cytometry analysis confirmed uptake of the fluorescent lipid probes was up to 5-fold higher in CLL cells compared to healthy B-lymphocytes. Uptake of the methyl ester was consistently higher compared to the carboxylic acid variant over 48 hours for the CLL cell lines. No significant change in proliferation was observed over 48 hours in the CLL cell lines; proliferation was slightly decreased in the healthy B-lymphocytes for both fluorescent lipids at concentrations above 5 µM. However, viability remained consistent over 48 hours for all cell lines at all lipid concentrations. Uptake of the fluorescent lipids in skeletal muscle cells was 2-fold lower compared to CLL cell lines but was approximately 1.5-fold higher than healthy B-lymphocytes. Early GCMS results have shown high uptake of the fluorinated lipids in CLL cell lines after 24 hours. Samples of healthy B-lymphocytes and skeletal muscle are being prepared at the time of application.
Discussion
Flow cytometry data revealed the CLL cell lines had significantly higher uptake of the fluorescent lipids compared to the skeletal muscle and healthy B-lymphocytes. These results are consistent with previously published data (Thurgood et al, Exp. Hematol. 2022) and could suggest the lipid PET tracer would be primarily sequestered by B-CLL cells in vivo, followed by minimal uptake into skeletal muscle. The CLL cells showed preference for the methyl ester derivative over the carboxylic acid, possibly due to the structure of the methyl ester being more amenable to transport and storage in the cell (Havel, Am J Physiol. 1987; Czech et al, Diabetologia. 2013). Cell proliferation did not change significantly over 48 hours, suggesting the lipids are not driving CLL cell growth. Microscopy experiments currently being optimised will help elucidate the fate of the lipids inside the cell (e.g. metabolism, storage). Early GCMS results corroborate the flow cytometry data, demonstrating high uptake of the lipid probes in the CLL cell lines, however, uptake is yet to be confirmed in healthy B-lymphocytes and skeletal muscle.
Conclusions
We hypothesised that a lipid PET tracer would be more informative for CLL diagnosis and surveillance than the FDG-PET tracer. Flow cytometry (and GCMS) data has shown high uptake of novel lipid probes in CLL cell lines, with minimal uptake in healthy B-lymphocytes and skeletal muscle. Additionally, this project has established a pipeline for the development of novel lipid PET tracers that could be applied to other lipid-avid malignancies.
Keywords : leukemia, lipid, imaging
Please indicate how this research was funded. : Funding for this project was obtained through a seed grant.
Please indicate the name of the funding organization.: Flinders Foundation