Authors
Riccardo Moia, Chiara Cosentino, Samir Mouhssine, Antonella Zucchetto, Ilaria Romano, Matin Salehi, Luca Vincenzo Cappelli, Fabio Iannelli, Mohammad Almasri, Nawar Maher,1 Lorenzo Fumagalli, Deborah Cardinali, Andrea Visentin, Jana Nabki, Luca Cividini, Bashar Al Deeban, Milena Lazzaro, Francesca Maiellaro, Annalisa Gaglio, Francesca Perutelli, Valentina Griggio, Riccardo Dondolin, Matteo Bellia, Maura Nicolosi, Silvia Rasi,1 Eleonora Secomandi, Valeria Caneparo, Abdurraouf Mokhtar Mahmoud, Clara Deambrogi, Sreekar Kogila, Joseph Ghanej, Mohammad Reshad Nawabi, Ilaria Del Giudice, Elisa Albi, Candida Vitale, Lydia Scarfò, Marta Coscia, Livio Trentin, Stefano Pileri, Paolo Ghia, Roberto Chiarle, Valter Gattei, Lodovico Terzi di Bergamo, Davide Rossi, Robin Foà, Gianluca Gaidano.
Background
Clonal hematopoiesis (CH) is the clonal expansion of a hematopoietic stem cell in otherwise healthy individuals, due to somatic driver mutations in genes mainly implicated in myeloid neoplasms. The clinical and biological significance of CH has not been investigated in the myeloid compartment of chronic lymphocytic leukemia (CLL).
Methods
Genomic DNA was extracted from granulocytes of CLL patients referring at our institution at the time of diagnosis. Multicenter cohorts of CLL patients with available granulocytes before and after fixed-duration venetoclax-based therapy and BTK inhibitors were analyzed. Samples were investigated by targeted next-generation sequencing (NGS), employing a custom panel of genes (N=28) recurrently mutated in CH. Cell sorting, single cell (sc) DNA and scRNA sequencing were also performed in cases provided with viable cells.
Results
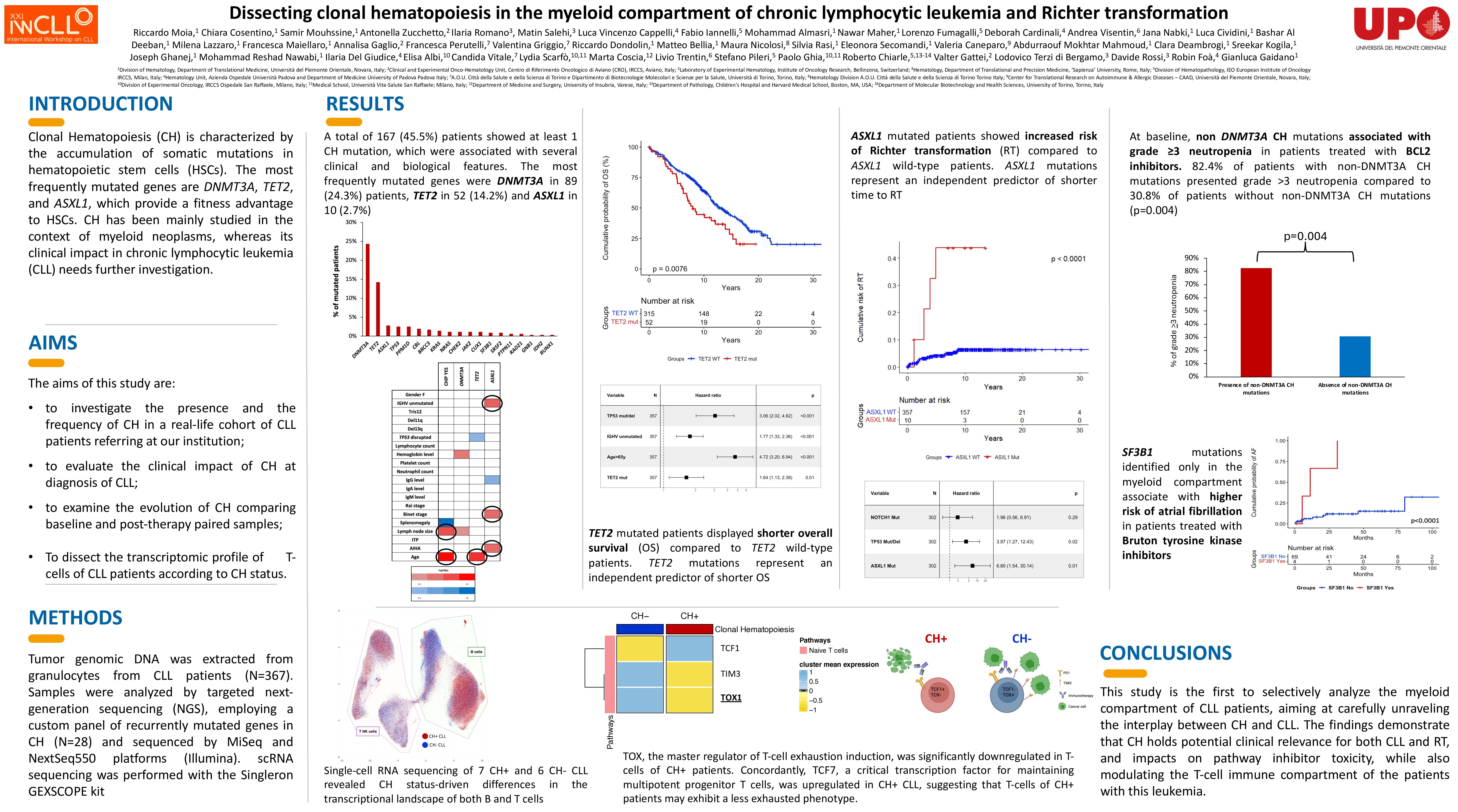
A total of 488 consecutive CLL have been included in the present study. Overall, at least 1 CH mutation was identified in 231 (47.3%) patients. The most frequent mutations identified on granulocytes affected DNMT3A in 129 (26.4%) patients, followed by TET2 in 74 (15.2%), ASXL1 in 15 (3.1%), TP53 in 13 (2.7%), and PPM1D in 11 (2.3%). To evaluate the distribution of CH mutations across different peripheral blood cell compartments, 33 CH+ patients who never developed a Richter transformation (RT) were sorted by FACS and the CLL compartment (CD19+/CD5+ cells), the T-cell compartment (CD3+ cells) and the myelomonocytic compartment (CD14+ cells) were independently sequenced. Overall, the mutational analysis of CH in sorted cells showed that CH mutations are not present in the CLL compartment but specifically localize in the myelomonocytic compartment. In addition, DNMT3A mutations in 12 patients and TET2 mutations in 1 patient were also detected in the T-cell compartment, confirming the possible lympho-monocyte skewage of some CH variants. This analysis also allowed to evaluate the distribution of TP53 variants in different cell compartments. In 5 patients, TP53 mutations were found exclusively in the myelomonocytic compartment (i.e. CD14+ cells), therefore representing CH-related, and not CLL-related, TP53 variants. After a median FU of 12.3 years, the presence of at least one CH mutation significantly associated with shorter OS (10.8 years vs 13.6 years, p=0.023). In particular, TET2 mutations retained an independent association with shorter OS (HR 1.53, p=0.01) when adjusted for age, IGHV and TP53 status. The presence of CH also predisposed to a higher risk of second hematological malignancies, that developed in 9 CH+ patients and in only 1 CH- patient, accounting for a 20-year risk of developing a second hematological malignancy of 30.9% for CH+ patients compared to 1.2% for CH- patients (p=0.0004). By analyzing 30 patients treated with venetoclax-based therapy, CH predicted higher risk of grade >3 neutropenia (p=0.004). In patients receiving continuous BTKi (N=73), CH SF3B1 mutations (HR 9.75, p=0.003) associated with an increased risk of atrial fibrillation when adjusted for age and other cardiovascular comorbidities. scRNA sequencing of 7 CH+ compared to 6 CH- CLL (matched for FISH, IGHV and TP53 status) revealed that CH influences the transcriptomic profiles of both B and T cells. CH+ patients displayed cell pathways indicative of heightened T-cell activation and a more pro-inflammatory profile, corroborated also by increased serum levels of CCL3 and CCL4. T-cells of CH+ patients exhibit a less exhausted phenotype, as documented by a lower expression of TOX, the master regulator of T-cell exhaustion induction. By evaluating RT, ASXL1 mutations associated with a higher risk of RT (HR 6.80, p=0.01) also when adjusted for the TP53 and NOTCH1 status. Interestingly, in 2 patients who subsequently developed RT, scDNA sequencing identified CH mutations also in CLL cells.
Conclusions
CH in CLL harbors potential clinical relevance for both CLL and RT, while also influencing the T-cell immune compartment.
Keywords : Clonal hematopoiesis, Richter Transformation
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: This work was supported by “Molecular bases of disease dissemination in lymphoid malignancies to optimize curative therapeutic strategies” (5 x 1000 No. 21198), Associazione Italiana per la Ricerca sul Cancro (AIRC) Foundation Milan, Italy; the AGING Project – Department of Excellence – DIMET, Università del Piemonte Orientale, Novara, Italy; PNRR-MAD-2022-12375673 (Next Generation EU, M6/C2_CALL 2022), Italian MoH, Rome, Italy; AIL Novara VCO ODV, Novara, Italy.