Authors
Víctor Arenas Gutiérrez, Jose Luis Castaño, Daniela Vallejo Iriarte, Juan Jose Dominguez Garcia, Lucrecia Yáñez San Segundo and Carlos Pipaón Gonzalez
Introduction
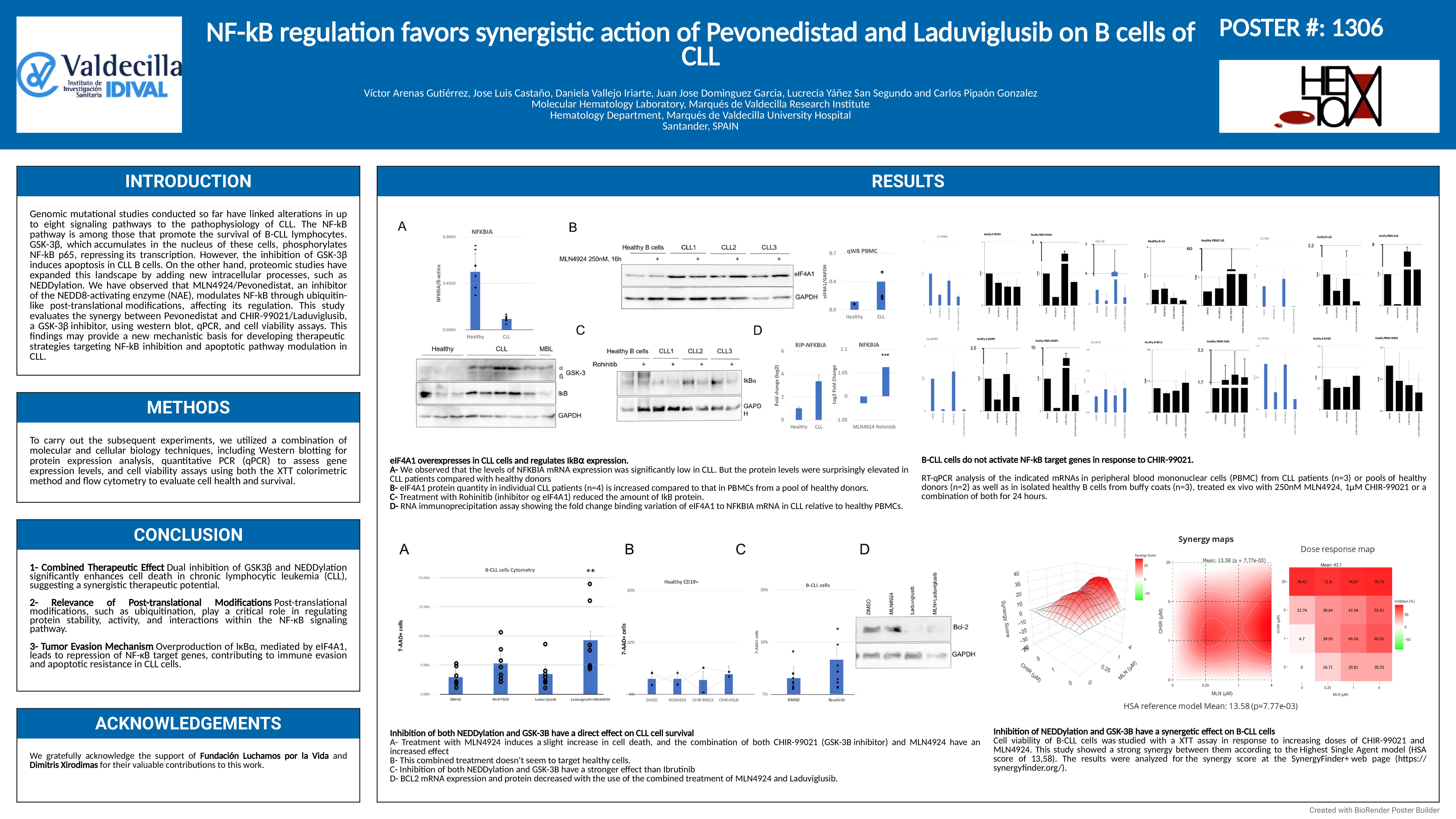
The causes of chronic lymphocytic leukemia (CLL) remain largely unknown to this day. Characteristically, B cells in CLL exhibit prolonged survival, partly due to the activation of survival signaling pathways, such as NF-kB. This pathway is activated by extracellular signals and intercellular contacts. Additionally, other signaling pathways can modulate NF-kB-dependent transcription. GSK-3β, for example, which accumulates in the nucleus of CLL B cells, can phosphorylate the p65 subunit of NF-kB, turning it into a transcriptional repressor. However, it has been reported that its inhibition induces cell death in CLL B cells.
To prevent continued signaling in healthy cells, the NF-kB pathway activates the transcription of its own inhibitor, IkBα, whose stability mediated by the ubiquitin-proteasome system is the central mechanism by which the pathway is regulated. Because of this, several groups have explored a possible way to inhibit this pathway through post-translational modifications like ubiquitination. Thus, it was found that NF-kB is a key target of a NEDD8-activating enzyme (NAE) inhibitor, related to the ubiquitin signaling cascade, Pevonedistat.
Objective
The aim of this study is to evaluate the possible synergies of Pevonedistat with other agents targeting NF-kB, in order to understand the regulation of this survival pathway in CLL and explore new therapeutic strategies for its treatment.
Methods
Using western blot and qPCR, along with cell viability assays, including flow cytometry with 7AAD staining and XTT, we evaluated the cytotoxic effects of treatments with Pevonedistat and Laduviglusib.
Results
We studied various mechanisms that modulate NF-kB signaling in B-CLL cells. We found a discrepancy between the mRNA and protein levels of IkBα. Our observations revealed a translational control of its expression not previously described in these cells. The high translation of IkBα mRNA would contribute to the tumor B lymphocytes in CLL not inducing the NF-kB pathway in response to GSK-3β inhibition, which does occur in other peripheral blood cells from these patients.
Given this, we decided to study the effect of the combined inhibition of GSK-3β and ubiquitin-like post-translational modifications, both elevated in CLL cells. We observed a synergistic effect between the specific GSK-3β inhibitor, Laduviglusib, and the NEDD8-activating enzyme inhibitor, Pevonedistat, on B cells from CLL patients treated ex vivo. Since the action on the NF-kB pathway alone did not seem to explain the cytotoxic effect of this combination, we conducted a study of the variations in the transcriptome of tumor B cells from CLL in response to treatment. The results indicate that the combined treatment enhances the reduction of BCL2 mRNA levels, suggesting an alternative pathway for the inhibition of BCL-2 in CLL. This finding could have therapeutic implications for the treatment of refractory cases of CLL, as it is capable of enhancing the action of Venetoclax at low concentrations. Additionally, we also observed a significant decrease in the expression of BIRC3 mRNA, an important anti-apoptotic factor, but whose mutation in CLL paradoxically is associated with a worse prognosis.
Conclusions
In this work, we report a novel translational regulation of IkBα in CLL B cells that explains the lack of correlation between the levels of its mRNA and protein and provides a rational basis for the use of GSK-3β inhibitors in these cells. We found a synergy in the ex vivo combinational treatment with Laduviglusib and Pevonedistat. Finally, we provide evidence of a collaborative inhibition of BCL-2 expression by this combined treatment, which offers new mechanistic support for its cytotoxic action.
Keywords : NEDDylation, GSK-3ß, RNA translation
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: