Authors
A.M. White, O.G. Best, B.J. Kuss, L.A. Thurgood
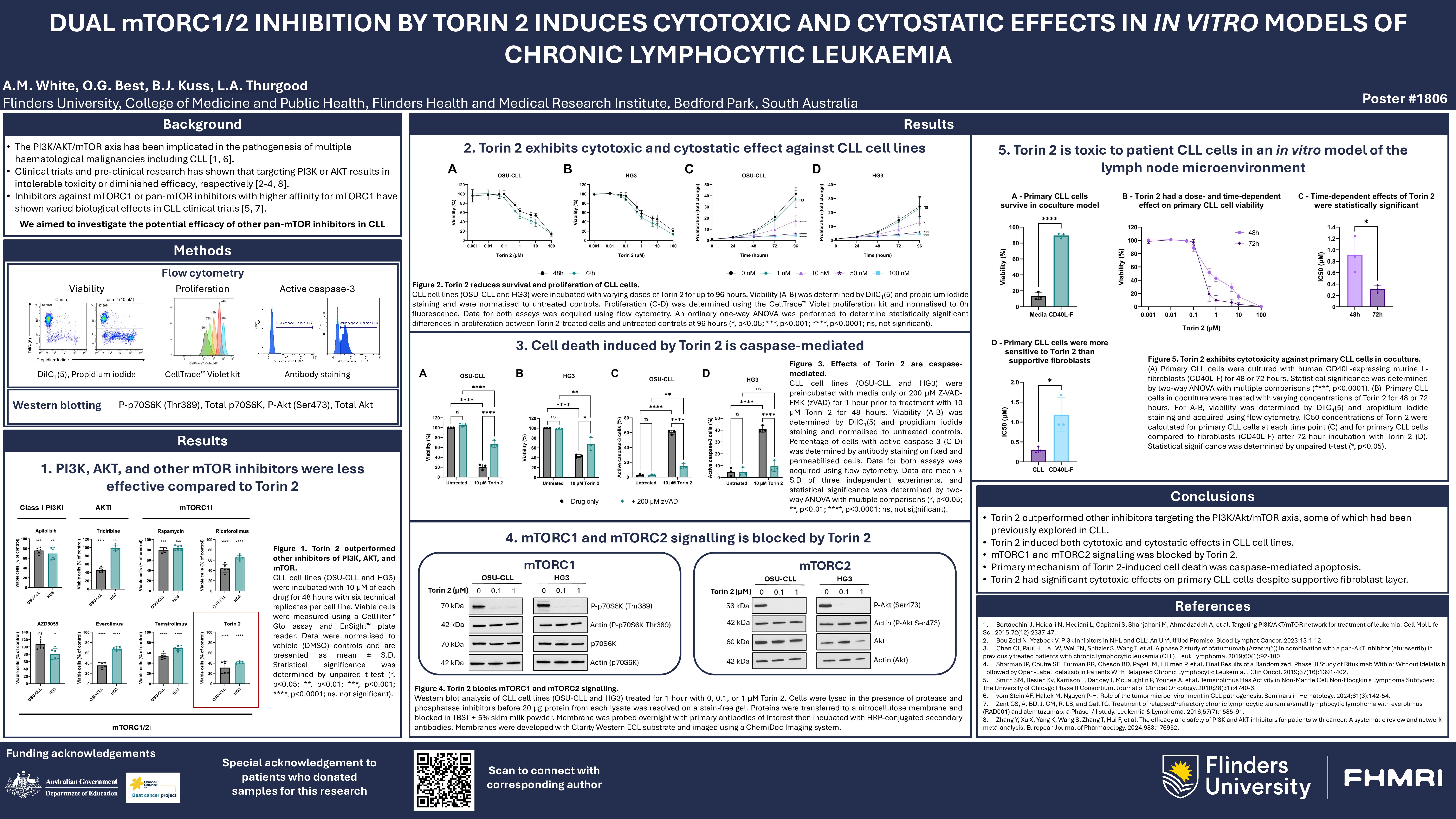
Background
Targeted therapies have transformed the management of chronic lymphocytic leukaemia (CLL), yet relapse and drug resistance remain prevalent. The PI3K/AKT/mTOR axis is constitutively active in CLL cells and supports their survival within the tumour microenvironment (TME). While PI3K inhibitors have shown promise, their long-term use is hampered by toxicity and resistance. mTOR, a key downstream effector, exists as two complexes: mTORC1 and mTORC2. Previous studies have shown that mTORC1-specific inhibitors lacked efficacy in CLL, suggesting dual inhibition of both mTOR complexes may be more effective. Torin 2 is a potent inhibitor of both mTORC 1 and 2 and has demonstrated efficacy in various cancers. This study evaluated the cytotoxic and cytostatic potential of Torin 2 in in vitro CLL models.
Methods
We conducted a large, high-throughput screen of 397 compounds, including a range of mTORC1, mTORC2 and dual mTOR inhibitors. CLL cell lines (HG3 and OSU-CLL) were treated with 10 µM of the compounds for 48 hours and their viability assessed using the CellTiter-Glo luminescent assay (n=6 technical replicates per condition). For detailed characterisation of lead compounds, dose- and time-response studies were conducted using 0.001–100 µM Torin 2 over 48 and 72 hours. Proliferation was assessed by CellTrace Violet dye dilution. Data were normalized to baseline mean fluorescence intensity (MFI), with statistical analysis performed using a one-way ANOVA with Dunnett’s correction. Western blotting was used to evaluate the phosphorylation status of mTOR downstream targets (p70S6K at Thr389 for mTORC1; AKT at Ser473 and Thr308 for mTORC2) following Torin 2 treatment for 1, 6, or 24 hours. To elucidate the mechanism of action, cytotoxicity assays were performed with or without the apoptosis inhibitor z-VAD-FMK and the autophagy inhibitor Bafilomycin A1. Finally, primary CLL cells (n=4 at 48h; n=3 at 72h) were treated with Torin 2 while in co-culture with stromal cell monolayers to mimic the TME.
Results
Data from the high throughput screen determined that Torin 2 was significantly more effective at reducing CLL cell viability than the PI3K, AKT, mTORC1-selective and alternate pan-mTOR inhibitors. Torin 2 reduced cell viability below 50% in both the HG3 and OSU-CLL lines (p < 0.01, Welch’s t-test). Independent experiments confirmed that low micromolar concentrations (≤5µM) were sufficient to induce significant time- and dose-dependent cytotoxicity on both lines. Proliferation assays also revealed potent cytostatic effects of Torin 2 in both CLL cell lines, with significant growth arrest observed following treatment with low nanomolar concentrations. Western blot analyses confirmed inhibition of mTORC1 by ablation of p70S6K phosphorylation and demonstrated suppression of mTORC2 through reduced phosphorylation of AKT at Ser473 and Thr308. Treatment with z-VAD-FMK or Bafilomycin A1 partially inhibited Torin 2-induced cell death, suggesting that both apoptosis and autophagy may be involved in mediating the cytotoxic effects of the drug. Torin 2 also significantly reduced the viability of primary CLL cells cultured with stromal cells (mean decrease >30%, p< 0.05, Welch’s one-tailed t-test), confirming efficacy of the drug under in vitro conditions that mimic the TME.
Discussion
The findings of this study highlight Torin 2 as a potent inhibitor of CLL cell viability and proliferation. Unlike earlier generation mTORC1-specific agents, Torin 2 targets both mTOR complexes, which may be the underlying reason for the enhanced anti-leukemic activity observed. Mechanistically, the dual cytotoxic and cytostatic effects of Torin 2 appear to involve apoptosis and autophagy, consistent with the role of mTOR in cellular metabolism and survival. The ability of Torin 2 to overcome the pro-survival effects of the interaction between CLL and stromal cells is particularly encouraging, as apoptosis resistance conferred by the TME is a major clinical challenge in CLL. These results suggest further pre-clinical studies of Torin 2 are warranted, both as a single agent and in combination with other targeted agents.
Conclusion
Torin 2 has significant anti-leukemic effects in vitro by blocking signalling via both mTORC1 and mTORC2. The cytostatic and cytotoxic activity of the agent, even under in vitro conditions that mimic the TME, provide a strong rationale for further preclinical development of Torin 2 as a therapeutic strategy for patients with CLL.
Keywords : mTOR inhibitor, Torin 2, autophagy
Please indicate how this research was funded. : Both internal and external grant organisations.
Please indicate the name of the funding organization.: Flinders Foundation and The Beat Cancer Project which was funded by Cancer Council South Australia and the South Australian Government.