Authors
Mazyar Shadman, Constantine S. Tam, Danielle M. Brander, Nataliya Kuptsova-Clarkson, Tian Tian, Marcus Lefebure, Jamie Hirata, Talha Munir.
Background
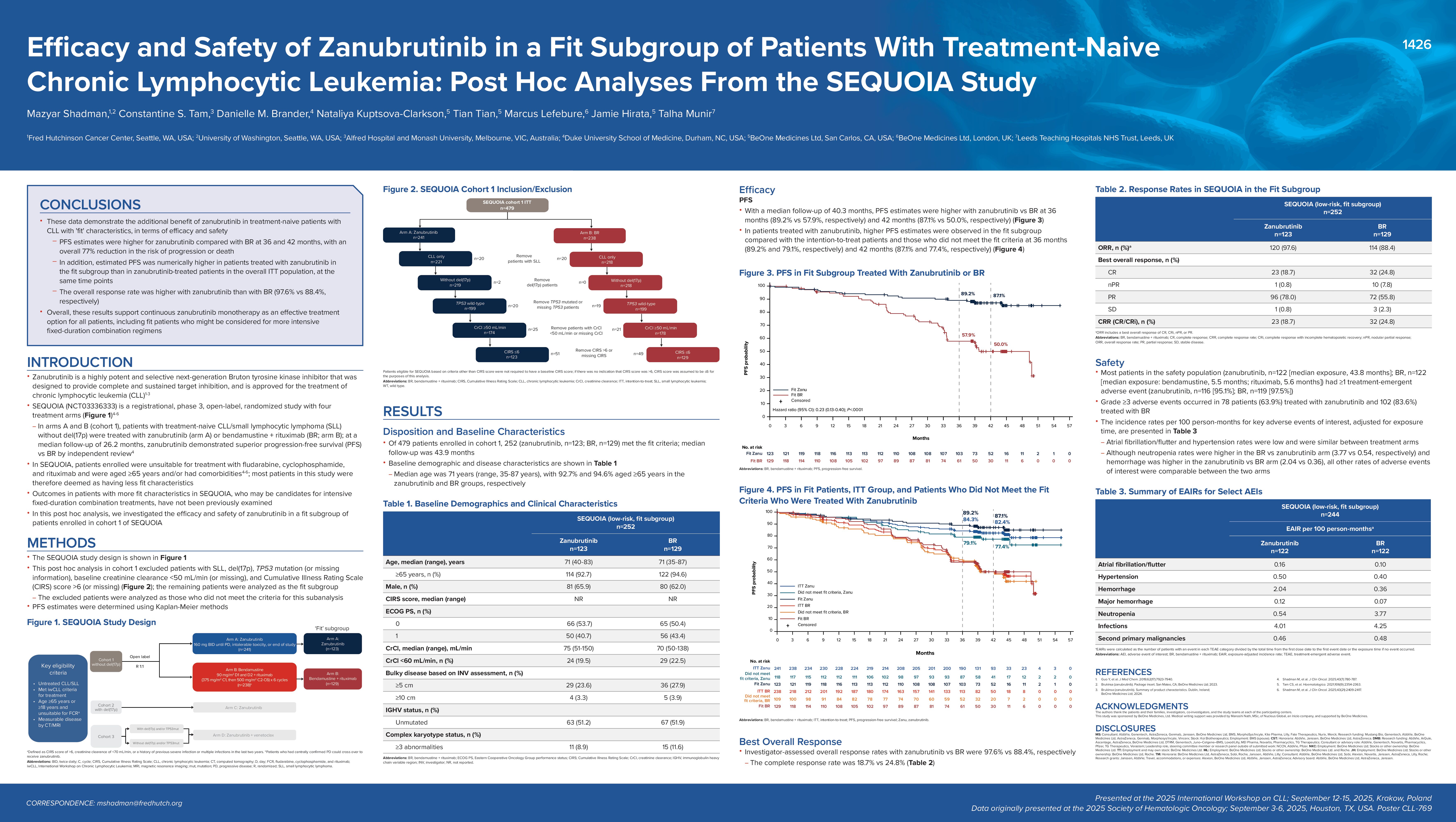
Zanubrutinib, a highly potent and selective next-generation Bruton tyrosine kinase (BTK) inhibitor, is approved for chronic lymphocytic leukemia (CLL). SEQUOIA (NCT03336333) is a phase 3 study comparing zanubrutinib with bendamustine-rituximab (BR) in patients with treatment-naive CLL/small lymphocytic lymphoma (SLL) without del(17p) who were considered unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab by age ≥65 years and/or comorbidities (Tam CS, et al. Lancet Oncology 2022;23(8):1031-1043). Outcomes among “fit” patients in this study who may be candidates for more intensive fixed-duration combination treatments were not previously examined.
Objective
To determine the efficacy and safety of zanubrutinib in a “fit” subgroup of patients without TP53-aberrations enrolled in SEQUOIA Cohort 1.
Methods
In this post hoc analysis, patients with SLL, del(17p), TP53 mutation (or missing), baseline creatinine clearance < 50 mL/min (or missing), and Cumulative Illness Rating Scale >6 were removed. The remaining patients were analyzed as the “fit” subgroup. Progression-free survival (PFS) estimates were determined using Kaplan–Meier methods.
Results
Of 479 patients enrolled in Cohort 1, 252 patients (53%; zanubrutinib, n=123; BR n=129) met the fit criteria. Median follow-up (range) was 43.9 (0.0-56.7) months. Median age was 71 (35-87) years, with 92.7% and 94.6% aged ≥65 years in zanubrutinib vs BR, respectively. The PFS estimates for zanubrutinib vs BR were 89.2% (95% CI, 82.1-93.6) vs 57.9% (95% CI, 48.2-66.5), respectively, at 36 months and 87.1% (95% CI, 79.5-92.1) vs 50.0% (95% CI, 39.8-59.4), respectively, at 42 months. Investigator-assessed overall response rates with zanubrutinib vs BR were 97.6% vs 88.4%. With median safety follow-up time of 44.0 months for zanubrutinib and 39.2 months for BR, the incidence rates adjusted for follow-up time for key events of interest (EOI) of any grade per 100 person-months were atrial fibrillation/flutter (0.16 vs 0.10), hypertension (0.50 vs 0.40), hemorrhage (2.04 vs 0.36), major hemorrhage (0.12 vs 0.07), neutropenia (0.54 vs 3.77), infections (4.01 vs 4.25), and second malignancies (0.46 vs 0.48); the adjusted rates for ≥Grade 3 EOI were atrial fibrillation/flutter (0.06 vs 0.05), hypertension (0.28 vs 0.20), neutropenia (0.41 vs 2.99), infections (0.64 vs 0.87), and second malignancies (0.14 vs 0.20).
Conclusions
These data demonstrate that zanubrutinib is effective and safe in fit patients. The estimated 36-month PFS outcomes appear favorable compared to those reported in contemporary studies enriched for younger, fit patients. These results support continuous zanubrutinib monotherapy as an effective treatment option for all patients, including fit patients who might otherwise be considered for more intensive fixed-duration combination regimens.
Keywords : Zanubrutinib, Bruton tyrosine kinase inhibitor, CLL/SLL
Please indicate how this research was funded.:
Please indicate the name of the funding organization. : BeOne Medicines Ltd