Authors
Lauren Stott, Eun Young Han, Iona Ashworth, Emma Kennedy, Eleanor Farrant-Jayawant, Aimilia Vareli, John Jones, Eleni Ladikou, Rosalynd Johnston, Fabio Simoes, Simon Mitchell, Shudong Wang, Chris Pepper, Andrea Pepper.
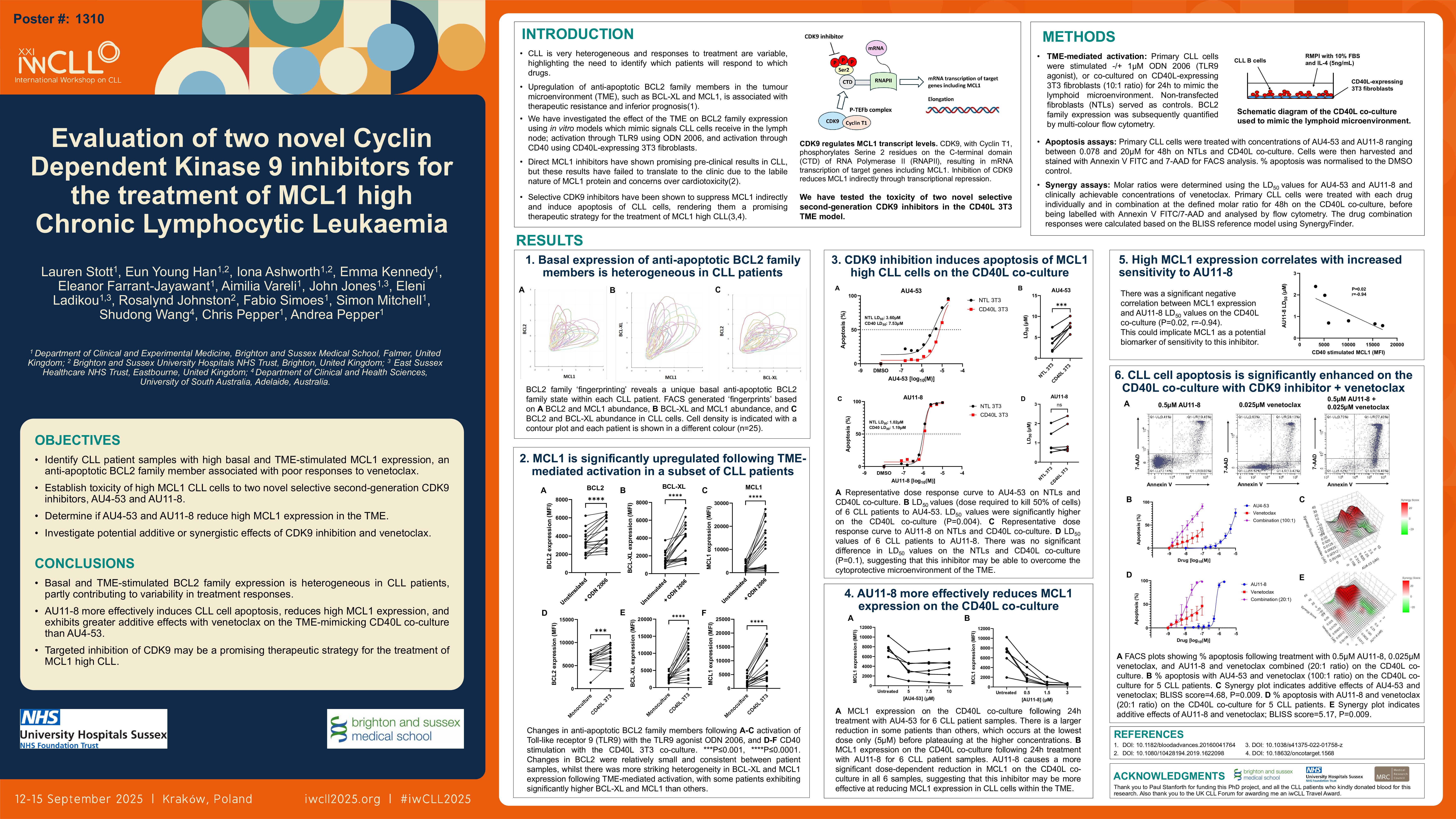
Background
Despite the introduction of BTK and BCL2 targeted drugs, chronic lymphocytic leukaemia (CLL) remains largely incurable, and patients who become double refractory to these inhibitors have limited options representing an emerging clinical challenge. Clinical responses are highly variable in CLL highlighting the need to predict which patients will respond to which drugs.
BCL-XL and MCL1, members of the BCL2 family of anti-apoptotic proteins, play a crucial role in CLL cell survival and their compensatory upregulation in the lymphoid microenvironment is known to confer resistance to the BCL2 inhibitor venetoclax (Thijssen et al, 2022). Dual inhibition of BCL2 and MCL1/BCL-XL could be a promising therapeutic strategy to overcome venetoclax resistance in CLL. Direct MCL1 inhibitors have shown promising preclinical results in CLL (Wei et al, 2020), but these results have failed to translate to the clinic due to the labile nature of MCL1 protein and concerns over cardiotoxicity (Wang et al, 2013). However, MCL1 protein levels can also be decreased indirectly by inhibiting transcriptional cyclin dependent kinases (CDKs).
CDK9 promotes transcription elongation via RNA Polymerase II phosphorylation which rapidly increases the abundance of short-lived proteins, including MCL1 (Shapiro, 2006). The CDK9 inhibitor VIP152 has been shown to significantly reduce disease burden and improve overall survival in a murine CLL model (Sher et al, 2022), providing a rationale for the use of CDK9 inhibitors in CLL.
We evaluated two novel selective CDK9 inhibitors, AU4-53 and AU11-8, for the treatment of CLL and hypothesised that patients with high MCL1 expression will be particularly responsive and that patients with high BCL2 and MCL1 expression may benefit from combination therapy with venetoclax.
Methods
Peripheral blood expression of BCL2, BCL-XL and MCL1 was measured in primary CLL cells by multi-colour flow cytometry. To mimic interactions of CLL cells in the lymphoid microenvironment where they are known to upregulate BCL-XL and MCL1 and show marked resistance to venetoclax, primary CLL cells were stimulated using; anti-IgM-coated dynabeads to activate the B cell receptor (BCR), ± 1μM ODN 2006 to activate Toll-like receptor 9 (TLR9), or co-cultured with NIH3T3 fibroblasts expressing human CD40L or untransfected cells (NTLs). CD40L mimics the stimulation CLL cells receive from activated T-cells in the lymph nodes. CLL cell viability was measured ±CD40L co-culture by flow cytometry using Annexin V/7-AAD staining. Subsequently, MCL1 protein expression and synergy between AU4-53/AU11-8 and venetoclax was assessed under these cytoprotective conditions.
Results
We observed heterogeneous basal expression of BCL2, BCL-XL and MCL1 in primary CLL cells (n=25). Stimulation of primary CLL cells through the BCR, TLR9 and CD40 receptor to mimic the lymphoid niche induced small average fold-increases in BCL2 (BCR 1.10, TLR9 1.39, CD40 1.26, n=18), but larger average fold-increases in BCL-XL (BCR; 1.45, TLR9; 2.25, CD40; 3.15, n=18) and MCL1 (BCR; 4.96, TLR9; 4.23, CD40; 3.35, n=18). Despite this, MCL1 expression remained extremely heterogeneous, and CLL cells which upregulated this protein did so consistently within all three in vitro stimulation models. From this we have identified patients whose CLL cells appear strongly dependent on MCL1 in the lymphoid microenvironment and are potentially more likely to be venetoclax resistant.
In all six primary CLL samples tested, the CDK9 inhibitors AU4-53 and AU11-8 effectively induced apoptosis of MCL1 high CLL cells on the CD40L co-culture, indicating their potential to overcome the cytoprotective lymphoid microenvironment. CLL cells most sensitive to AU11-8 had greater MCL1 expression (P=0.02, r=-0.94), implicating MCL1 as a potential biomarker of sensitivity to this inhibitor. Both AU4-53 and AU11-8 caused a dose-dependent reduction in MCL1 protein expression on the CD40L co-culture in all three primary CLL samples tested so far, and preliminary data suggests that both inhibitors may show potential to synergise with venetoclax under these cytoprotective conditions.
Conclusion
The novel selective CDK9 inhibitors AU4-53 and AU11-8 show in vitro efficacy in CLL, implicating targeted inhibition of this kinase as an exciting therapeutic strategy for the treatment of MCL1 high CLL.
Keywords : MCL1, CDK9, drug resistance
Please indicate how this research was funded. : Paul Stanforth PhD Scholarship and Brighton and Sussex Medical School
Please indicate the name of the funding organization.: Paul Stanforth PhD Scholarship and Brighton and Sussex Medical School