Authors
F. Perutelli, M. Arata, M.C. Montalbano, R. Minuto, M. Nicolosi, S. Pepe, M. Deodato, E. Fioribello, A. Ferrario, E. Boccellato, R. Moia, M. Bullo, D. Gottardi, M. Foglietta, C. Salvetti, R. Freilone, G. Gaidano, M. Coscia, A.M. Frustaci, F.R. Mauro, B. Bruno, C. Vitale.
Background
Treatment options for patients with chronic lymphocytic leukemia (CLL) have significantly increased over the years, with chemoimmunotherapy-free regimens now available from first-line therapy (2024 CLL ESMO guidelines). Selecting the optimal treatment requires careful consideration of multiple factors, including biological risk, side effect profiles, concomitant medications, patient fitness and individual preferences. In the absence of mature head-to-head comparisons, this decision-making process remains complex.
Aims
The FIRST-CLL study describes the allocation of first-line treatments for CLL in a real-world Italian cohort and explores the relative weight of several parameters – such as clinical-biological characteristics, logistical factors and patient preference – in the regimen selection.
Patients and methods
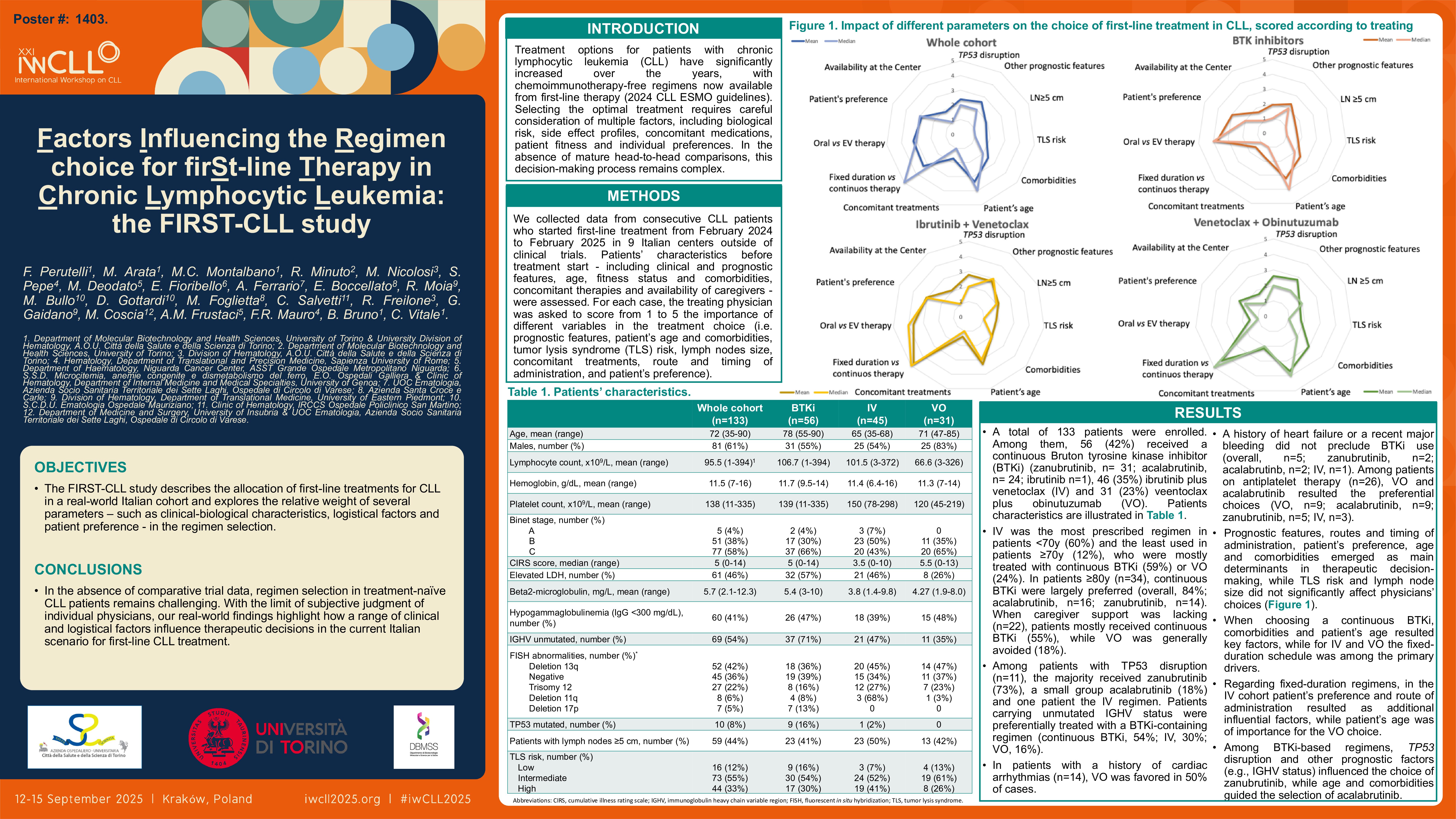
We collected data from consecutive CLL patients who started first-line treatment from February 2024 to February 2025 in 9 Italian centers outside of clinical trials. Patients’ characteristics before treatment start – including clinical and prognostic features, age, fitness status and comorbidities, concomitant therapies and availability of caregivers – were assessed. For each case, the treating physician was asked to score from 1 to 5 the importance of different variables in the treatment choice (i.e. prognostic features, patient’s age and comorbidities, tumor lysis syndrome (TLS) risk, lymph nodes size, concomitant treatments, route and timing of administration, and patient’s preference).
Results
A total of 133 patients were enrolled. Among them, 56 (42%) received a continuous Bruton tyrosine kinase inhibitor (BTKi) (zanubrutinib, n= 31; acalabrutinib, n= 24; ibrutinib n=1), 46 (35%) ibrutinib plus venetoclax (IV) and 31 (23%) venetoclax plus obinutuzumab (VO).
IV was the most prescribed regimen in patients < 70 years old (60%) and the least used in patients ≥70 years old (12%), who were mostly treated with continuous BTKi (59%) or VO (24%). In patients ≥80 years old (n=34), continuous BTKi were largely preferred (overall, 84%; acalabrutinib, n=16; zanubrutinib, n=14). When caregiver support was lacking (n=22), patients mostly received continuous BTKi (55%), while VO was generally avoided (18%).
Among patients with TP53 disruption (n=11), the majority received zanubrutinib (73%), a small group acalabrutinib (18%) and one patient the IV regimen. Patients carrying unmutated IGHV status were preferentially treated with a BTKi-containing regimen (continuous BTKi, 54%; IV, 30%; VO, 16%).
As expected, in patients with a history of cardiac arrhythmias (n=14), the treatment not including a BTKi (VO) was favored in 50% of cases. Interestingly, a history of heart failure or a recent major bleeding did not preclude BTKi use, although numbers were limited (overall, n=5; zanubrutinib, n=2; acalabrutinb, n=2; IV, n=1). Among patients on antiplatelet therapy (n=26), VO and acalabrutinib resulted the preferential choices (VO, n=9; acalabrutinib, n=9; zanubrutinib, n=5; IV, n=3).
We then analyzed the impact of each parameter on treatment selection, according to treating physicians, measured with the 1-to-5 scale. Overall, prognostic features, routes and timing of administration, patient’s preference, age and comorbidities emerged as main determinants in therapeutic decision-making (mean score: 2.61, 3.2, 3.3, 2.86, 3.36 and 2.44, respectively), while TLS risk and lymph node size did not significantly affect physicians’ choices (mean score: 1.77 and 1.88, respectively). When choosing a continuous BTKi, comorbidities and patient’s age resulted key factors (mean score: 2.96 and 3.67, respectively), while for IV and VO the fixed-duration schedule was among the primary drivers (mean score: 4.8 and 4.35, respectively). Regarding fixed-duration regimens, in the IV cohort patient’s preference and route of administration resulted as additional influential factors (mean score: 3.06 and 3.67, respectively), while patient’s age was of importance for the VO choice (mean score: 3.68).
Interestingly, when a continuous BTKi regimen was selected, TP53 disruption and other prognostic factors (e.g., IGHV status) had a different relevance for distinct drugs: for zanubrutinib mean scores were 2.13 and 2.55, respectively, whereas for acalabrutinib were 1.67 and 2.04, respectively. Conversely, patients’ age and comorbidities emerged as more important in the selection of acalabrutinib (mean score: 3.67 and 2.96, respectively) compared to zanubrutinib (mean score: 2.97 and 2.16, respectively).
Conclusions
In the absence of comparative trial data, regimen selection in treatment-naïve CLL patients remains challenging. With the limit of subjective judgment of individual physicians, our real-world findings highlight how a range of clinical and logistical factors influence therapeutic decisions in the current Italian scenario for first-line CLL treatment.
Keywords : CLL, front-line, Therapy
Please indicate how this research was funded. : This research received no funding
Please indicate the name of the funding organization.: