Authors
Yamit Shorer Arbel, Liron Yitzhak, Ben-Zion Katz, Yair Herishanu.
Background
Chronic lymphocytic leukemia (CLL) is marked by T-cell dysfunction, which results in immune suppression and inadequate antitumor immune responses. Hematopoietic progenitor kinase-1 (HPK-1) acts as a negative regulator of T-cell activity, and its inhibition has shown promise in improving T-cell functionality. While cellular therapies have proven effective in various lymphomas, initial CLL studies have been less
successful, largely due to the disease’s immunosuppressive characteristics.
Aims
This study aimed to explore the effectiveness of HPK-1 inhibition combined with CD20XCD3 bispecific antibodies as a treatment strategy for CLL.
Methods
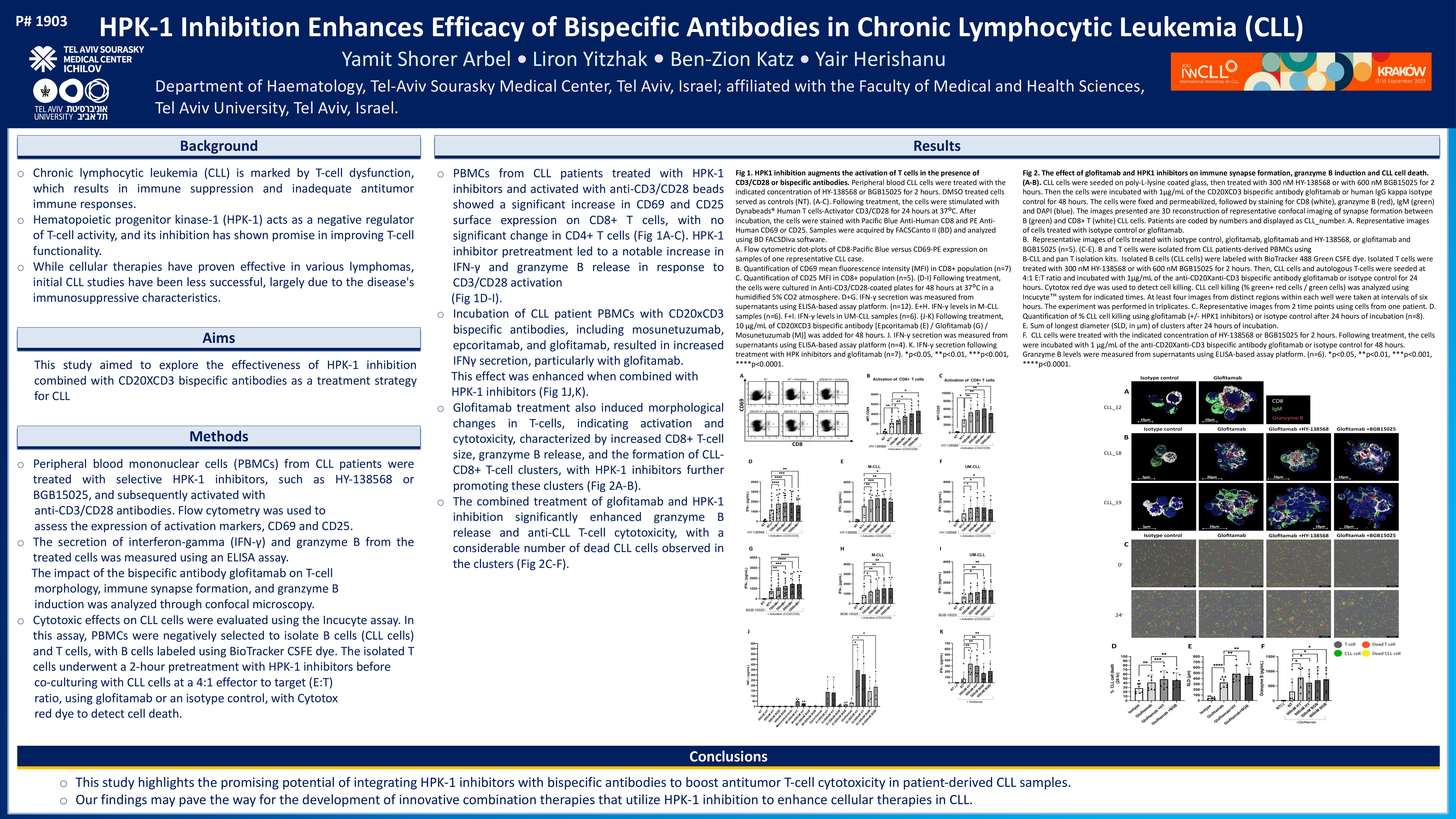
Peripheral blood mononuclear cells (PBMCs) from CLL patients were treated with selective HPK-1 inhibitors, such as HY-138568 or BGB15025, and subsequently activated with anti-CD3/CD28 antibodies. Flow cytometry was used to assess the expression of activation markers, CD69 and CD25. The secretion of interferon-gamma (IFN-γ) and granzyme B from the treated cells was measured using an ELISA assay. The impact of the bispecific antibody glofitamab on T-cell morphology, immune synapse formation, and granzyme B induction was analyzed through confocal microscopy, while cytotoxic effects on CLL cells were evaluated using the Incucyte assay. In this assay, PBMCs were negatively selected to isolate B cells (CLL cells) and T cells, with B cells labeled using BioTracker CSFE dye. The isolated T cells underwent a 2-
hour pretreatment with HPK-1 inhibitors before co-culturing with CLL cells at a 4:1 effector to target (E:T) ratio, using glofitamab or an isotype control, with Cytotox red dye to detect cell death.
Results
PBMCs from CLL patients treated with HPK-1 inhibitors and activated with anti-CD3/CD28 beads showed a significant increase in CD69 and CD25 surface expression on CD8+ T cells, with no significant change in CD4+ T cells. Additionally, HPK-1 inhibitor pretreatment led to a notable increase in IFN-γ and granzyme B release in response to CD3/CD28 activation. The incubation of CLL patient PBMCs with CD20xCD3 bispecific antibodies, including mosunetuzumab, epcoritamab, and glofitamab, resulted in increased IFNγ secretion, particularly with glofitamab. This effect was enhanced when combined with HPK-1 inhibitors. Glofitamab treatment also induced morphological changes in T-cells, indicating activation and cytotoxicity, characterized by increased CD8+ T-cell size, granzyme B release, and the formation of CLLCD8+ T-cell clusters, with HPK-1 inhibitors further promoting these clusters. The combined treatment of glofitamab and HPK-1 inhibition significantly enhanced granzyme B release and anti-CLL T-cell cytotoxicity, with a considerable number of dead CLL cells observed in the clusters.
Summary
This study highlights the promising potential of integrating HPK-1 inhibitors with bispecific antibodies to boost antitumor T-cell cytotoxicity in patient-derived CLL samples. Our findings may pave the way for the development of innovative combination therapies that utilize HPK-1 inhibition to enhance cellular therapies in CLL
Keywords : Bispecific, B-CLL, HPK-1
Please indicate how this research was funded. : Internal funding
Please indicate the name of the funding organization.: Tel Aviv Sourasky Medical center