Authors
Fen Zheng, Dongxia Qin, Dan Yang, Yun Liu, Huayuan Zhu, Juejin Wang.
Aims
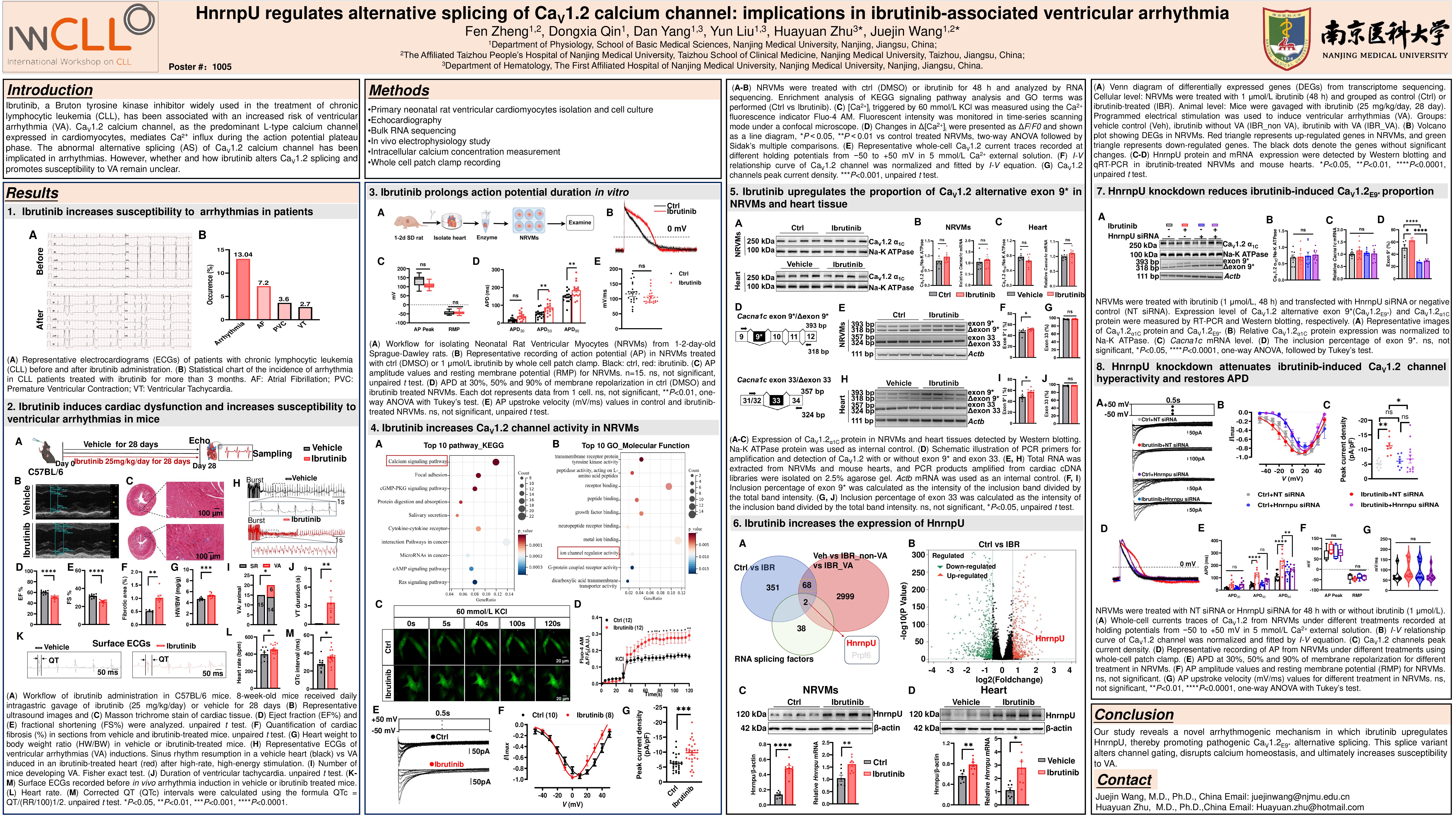
Ibrutinib, a Bruton tyrosine kinase inhibitor widely used in the treatment of chronic lymphocytic leukemia (CLL), has been associated with an increased risk of ventricular arrhythmia (VA). Alternative splicing (AS) of CaV1.2 calcium channel has been implicated in cardiac arrhythmias. This study aims to elucidate how ibrutinib alters CaV1.2 splicing and promotes VA susceptibility.
Methods
We conducted a retrospective analysis of clinical data from CLL patients treated with ibrutinib for ≥3 months at The First Affiliated Hospital of Nanjing Medical University between January and December 2023, focusing on electrocardiogram (ECG) parameters. To explore potential cardiotoxic mechanisms, 8-week-old male C57BL/6 mice were administered ibrutinib or vehicle at 25 mg/kg/day for 4 consecutive weeks via intragastric gavage. Cardiac function and morphology were assessed by echocardiography and Masson’s staining, respectively. Electrophysiological studies assessed VA inducibility in vivo. Neonatal rat ventricular myocytes (NRVMs) were used to examine action potential duration (APD), CaV1.2 channel function, and intracellular Ca²⁺ concentration ([Ca²⁺]i) via whole-cell patch clamp and Fluo-4 AM fluorescence imaging. Bulk RNA sequencing, RT-PCR and RNA immunoprecipitation (RIP) were employed to analyze AS events and protein-RNA interactions.
Results
76 CLL patients meeting the inclusion criteria were analyzed, and statistical analysis demonstrated that ~13% of these patients developed cardiac arrhythmias including VA after three months of continuous ibrutinib therapy. In mice, 4 weeks ibrutinib administration increases the susceptibility to ventricular arrhythmias to 30%. Bulk RNA sequencing revealed that ibrutinib significantly altered calcium signaling pathway and induced AS of Cacna1c. Indeed, ibrutinib increased the expression of the CaV1.2 alternative exon 9* (CaV1.2E9*) without affecting alternative exon 33. In NRVMs, ibrutinib hyperpolarized the current-voltage curve of cardiac CaV1.2 channels, prolonged APD, and raised K+-triggered [Ca2+]i. By using transcriptomics analysis, the splicing factor hnRNPU was found to be upregulated by ibrutinib treatment. RIP assay confirmed the direct binding of hnRNPU to CaV1.2 pre-mRNA. Knockdown of hnRNPU significantly reduced CaV1.2E9* expression, reversed the hyperpolarization trend of the CaV1.2 channel currents, and mitigated APD prolongation induced by ibrutinib.
Conclusion
Our study identifies a novel arrhythmogenic mechanism in which ibrutinib upregulates hnRNPU, promoting pathogenic CaV1.2E9* splicing. This aberrant splicing alters channel gating, disrupts calcium homeostasis, and ultimately increases susceptibility to VA.
Keywords : alternative splicing, Cav1.2 calcium channel, hnRNPU, Ibrutinib, ventricular arrhythmia.
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: