Authors
Talha Munir, Ph.D., Sean Girvan, M.Sc., David A Cairns, Ph.D., Adrian Bloor, Ph.D., F.R.C.Path., David Allsup, Ph.D., Abraham M. Varghese, Ph.D., Satyen Gohil, Ph.D., Shankara Paneesha, F.R.C.Path., Andrew Pettitt, F.R.C.Path., Toby Eyre, M.D., Christopher P. Fox, Ph.D., Francesco Forconi, F.R.C.Path., Constantine Balotis, M.D., Nicholas Pemberton, F.R.C.Path., Oonagh Sheehy, M.B., F.R.C.Path., John Gribben, M.D., D.Sc., Nagah Elmusharaf, F.R.C.Path., Simona Gatto, Ph.D., Gavin Preston, Ph.D., Anna Schuh, M.D., Renata Walewska, Ph.D., Lelia Duley, Nichola Webster, B.Sc., Surita Dalal, Ph.D., Andrew Rawstron, Ph.D., Dena Howard, Ph.D., Anna Hockaday, B.Sc., Sharon Jackson, Ph.D., Natasha Greatorex, B.Sc., Sue Bell, D.Phil., David Stones, M.Sc., Julia M Brown, M.Sc., Piers E.M. Patten, F.R.C.Path., Ph.D., Peter Hillmen, Ph.D.
Introduction
Ibrutinib (I) and venetoclax (V) improve CLL survival outcomes compared to chemoimmunotherapy. Mathematical disease modelling and Phase II studies favour defining treatment duration according to individual patient (pt) sensitivity to I+V combination. We hypothesized that I monotherapy or I+V where duration of therapy is guided by measurable residual disease (MRD) is more effective than FCR in CLL and that treatment duration personalised using MRD response would optimize outcomes.
Methods
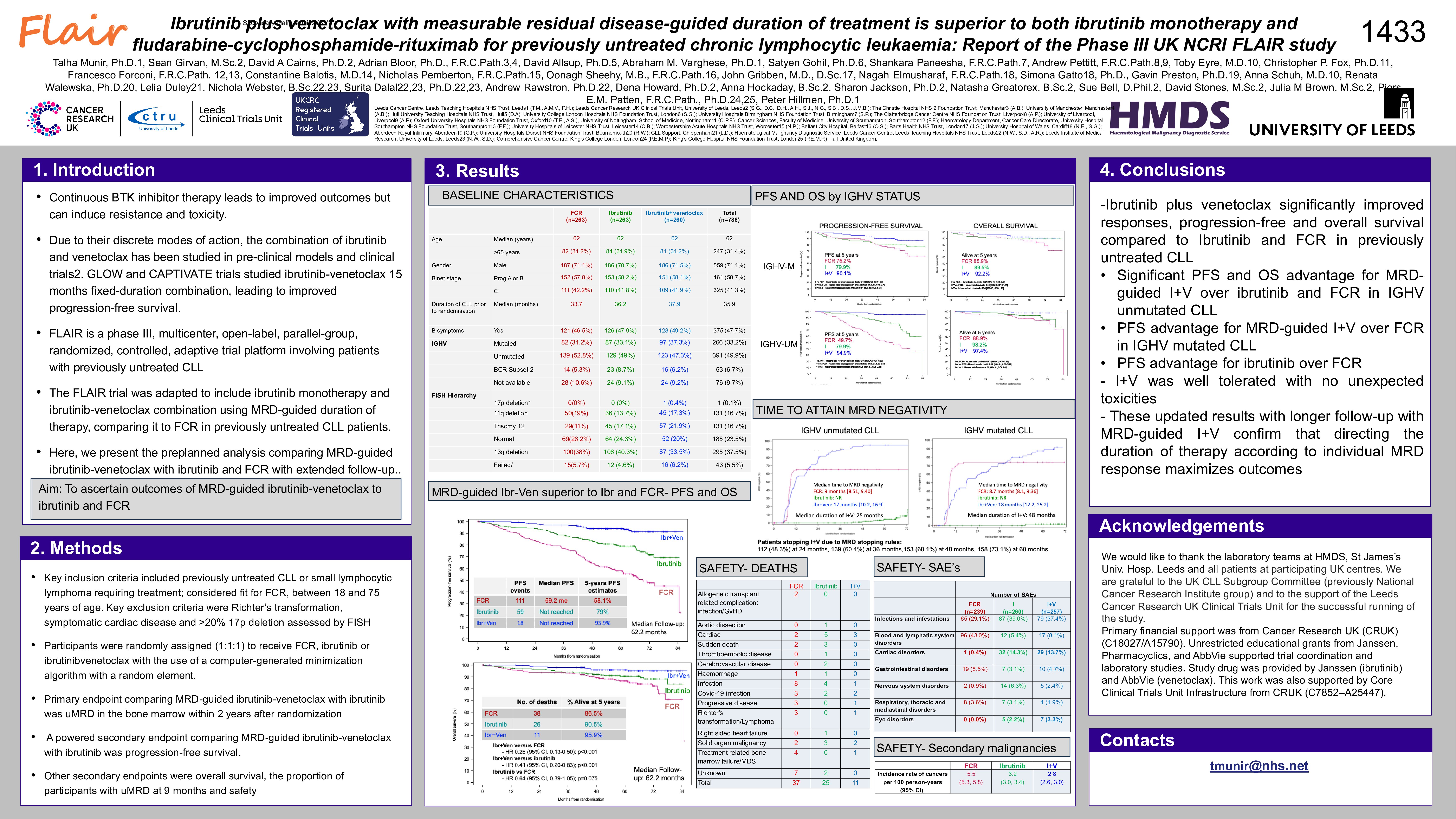
FLAIR (ISRCTN01844152) is an ongoing, phase III, multicentre, randomised, controlled, open, parallel group trial for pts with untreated CLL. We report a planned analysis of I+V vs I vs FCR. Peripheral blood (PB) MRD at a threshold of < 0.01% was assessed at 12 months and then 6 monthly. Time to undetectable MRD (uMRD) in the I+V and I monotherapy arms was calculated as date of therapy initiation to date of initial uMRD in PB, giving a treatment duration between 2 and 6 years. The primary endpoint for I+V vs I was uMRD in bone marrow (BM) up to 2 years. A powered secondary endpoint for I+V vs I vs FCR was progression-free survival (PFS), and other secondary endpoints were overall survival (OS), iwCLL response, MRD and safety.
Results
786 patients(pts) were randomised to FCR (n=263), I (n=263) or I+V (n=260) from 96 UK Centres from 20/07/2017 to 24/03/2021 with data-lock on 04/11/2024. 71.1% were male, median age was 62 years, 42% were Binet Stage C, IGHV unmutated 49.6%, 33% IGHV mutated and 6% Subset 2. Hierarchical FISH were 16.7% 11q del, 16.7% trisomy 12, 23.5% normal and 37.5% 13q del.
At two years, 299 participants were uMRD in BM: 127/263 in FCR (48.3), 0/263 in I only (0%) and 172/260 in I+V (66.2%) arms (p < 0.0001).
At a median of 62.2 months, there were 189 PFS events: 112 FCR, 59 I and 18 I+V. Hazard ratio (HR) for PFS for I+V vs I was 0.29 (95%CI: [0.17, 0.49]; p< 0.001). HR for PFS for I vs FCR was 0.44 (95% CI: [0.32, 0.60]; p< 0.001). HR for PFS for I+V vs FCR was 0.13 (95% CI: [0.08, 0.21]; p< 0.001). 5-year estimated PFS rates for FCR, I and I+V were 58.1%, 79.0%, 93.9% respectively. There were 76 deaths: 39 FCR, 26 I and 11 I+V. OS was significantly better for I+V vs. I (HR 0.41; 95% CI: [0.20,0.83]; p=0.013) with a trend towards improved OS for I vs. FCR (HR 0.64 (95% CI: [0.39, 1.05]; p=0.07). 5-year estimated OS rates for FCR, I and I+V were 88.2%, 91.3% and 96.2%.
At 9 months, uMRD in BM was higher in FCR arm as compared to I and I+V arm: 40.7% vs 0% vs 33.1%. However, with continued I+V more pts became uMRD in BM and PB. MRD stopping rules resulted in 115 participants stopping at 2 years, 25 participants stopping at 3 years and 13 participants stopping at 4 years. A higher proportion achieved an ORR for I and I+V; ORR 83.7% for FCR, 94.7% for I and 96.2% for I+V.
657 SAEs were reported in 390 (51.6%) pts (129 FCR vs 130 I vs 131 I+V). SAEs by organ class for FCR vs I vs I+V were infections in 18.8% vs 25.4% vs 23.7%; haematological in 31% vs 4.2% vs 5.8%; and cardiac in 0.4% vs 10.4% vs 10.9% respectively. 15 pts had sudden or cardiac deaths: 4 FCR, 8 I and 3 I+V. The incidence of other cancers per 100 pt-years was greater for FCR than I and I+V; 5.5 vs 3.2 vs 2.8.
Conclusion
I+V significantly improved uMRD, PFS and OS rates compared to I monotherapy and FCR in untreated CLL. FLAIR is the first trial confirming the superiority of MRD-guided I+V over I monotherapy and further substantiates the use of risk-adapted approach in untreated CLL patients to optimise outcomes.
Keywords : leukaemia, MRD, personalised
Please indicate how this research was funded.: Primary financial support was from Cancer Research UK (C18027/A15790). Unrestricted educational grants were provided by Johnson and Johnson, Pharmacyclics, and AbbVie.
Please indicate the name of the funding organization. : Cancer Research UK, Johnson and Johnson, Pharmacyclics, AbbVie.