Authors
Miroslav Boudny, Erika Bajusova, Pedro Faria Zeni, Daniel Filip, Eva Hoferkova, Laura Ondrisova, Josef Vecera, Kvetoslava Matulova, Sarka Pospisilova, Vaclav Seda, Petra Pavelkova, Krystof Hlavac, Nicolas Blavet, Leos Kren, Anna Panovska, Michael Doubek, Marek Mraz.
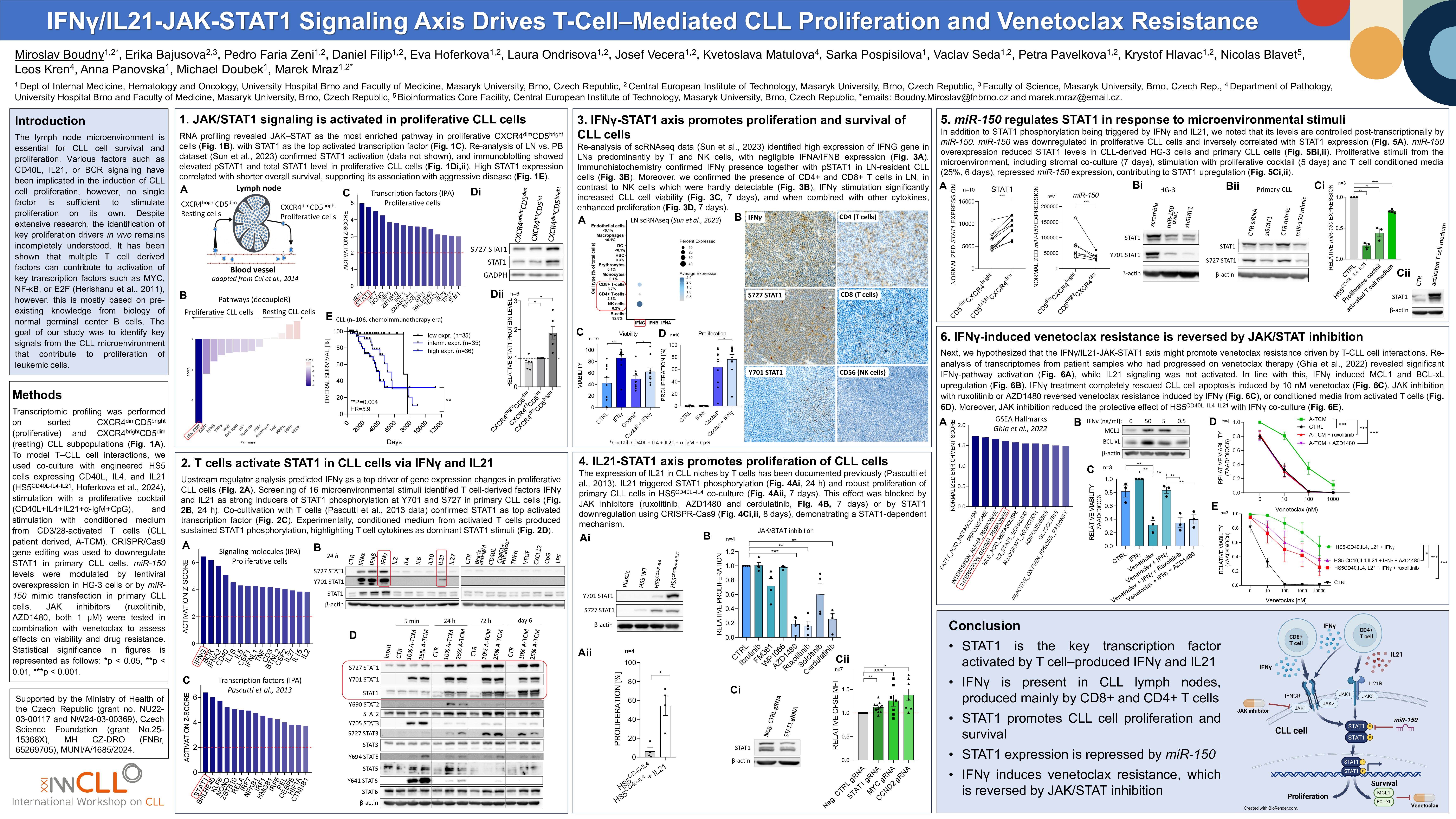
Background
Multiple microenvironmental factors have been implicated in the induction of CLL cell proliferation in lymph nodes. However, it remains unclear which signals are most essential and how they are (patho)physiologically combined to stimulate CLL cells into proliferation.
Results
To decipher the changes in the CLL microenvironment, we performed transcriptomic profiling (n=10 pairs) of resting (CXCR4brightCD5dim) and proliferative (CXCR4dimCD5bright) intraclonal CLL subpopulation that recently exited lymph nodes (LN). Subsequent gene expression analysis (decoupleR, IPA, GSEA) revealed the JAK-STAT as the most prominently activated pathway in CXCR4dimCD5bright cells, and specifically, the STAT1 transcription factor as most likely driving gene expression differences between CXCR4/CD5 subpopulations. The JAK-STAT1 signaling enrichment was confirmed in a re-analysis of RNA-seq from paired LN vs. peripheral blood samples (n=29 pairs, Sun et al., 2023). Notably, higher STAT1 mRNA levels are strongly associated with a shorter time to first treatment and overall survival in 2 CLL cohorts (n=106, in-house cohort; n=191, EGAS00001001746; both chemoimmunotherapy-era). Next, to identify STAT1 activator(s), we stimulated primary CLL cells (n=4) with a panel of 16 microenvironmental signals (anti-IgM, CD40L, CpG, CXCL12, IFNα/β/γ, IL2/4/6/10/21/27, LPS, TNFα, VEGF). IFNγ and IL21 emerged as the only inducers of STAT1 phosphorylation (Y701, S727), and IL21 also activated STAT3 in a phospho-protein analysis, but not other proteins (ELISA detecting 44 phospho-proteins). Indeed, in silico analysis (IPA) identified IFNγ and IL21 as receptor ligands most significantly influencing gene expression differences in LN-resident and CXCR4dimCD5bright CLL cells. We next observed that T-cells from CLL patients (CD3/28-activated) produce IFNγ (312 ng/ml) and IL21 (1 ng/ml), induce CLL proliferation, and long-term (6-day) STAT1 phosphorylation (STAT3 phosphorylated transitionally). The expression of IL21 in CLL niches has been documented (Pascutti et al., 2013), but the presence of IFNγ in CLL LNs seems counterintuitive considering the relatively immunologically “cold” microenvironment. We determined that IFNγ is clearly present in CLL LNs (n=8, immunohistochemistry), and CD8+ T-cells are its major source (scRNA-seq re-analysis, Sun et al., 2023). IFNγ stimulation (10 ng/ml) not only significantly enhanced CLL cell viability (42% vs. 86%, n=10, P< 0.001), but also increased CLL cell proliferation (64% vs. 76% proliferation, n=10, P=0.02) in combination with a cytokine cocktail (CD40L, IL4, IL21, anti-IgM, CpG). IL21 also increased proliferation in CLL cells co-cultured with HS5-CD40L-IL4 stromal cells (10% vs. 49%, P< 0.05, n=16; model by Hoferkova et al., 2024) while inhibition of JAK-STAT1 signaling by JAK-inhibitors (ruxolitinib or AZD1480) reduced proliferation by ~80% in the HS5-CD40L-IL4-IL21 co-cultures. Moreover, STAT1 knockout in primary CLL cells (Cas9-gRNA transfection) impaired proliferation induced by HS5-CD40L-IL4-IL21 co-culture (n=6; P< 0.05). In addition to STAT1 phosphorylation being triggered by IFNγ and IL21, we noted that its levels are controlled post-transcriptionally by miR-150. miR-150 overexpression (lentiviral) repressed STAT1 mRNA and protein levels in CLL-derived HG-3 and OSU-CLL cells (n>3) and in primary CLL cells transfected with synthetic miR-150 (n=5; P< 0.05). Indeed, miR-150 is downregulated, and STAT1 levels are upregulated in LN-derived CLL cells (1.9- and 1.5-fold, respectively, n=10, P< 0.001). We further hypothesized that the IFNγ/IL21-JAK-STAT1 axis might promote venetoclax resistance driven by CLL-T cell interactions. Supporting this, re-analysis of transcriptomes from samples obtained before venetoclax therapy and at relapse (n=7, Ghia et al., 2022) revealed significant IFNγ-pathway activation (GSEA, P=0.018), while IL21 signaling was not activated. In line with this, IFNγ (50 ng/ml) induced MCL1 and BCLxL upregulation in CLL cells (~2.4- and 2.0-fold, respectively; P< 0.05). IFNγ treatment completely rescued CLL cell apoptosis induced by 10 nM venetoclax (viability 56% vs. 100%, n=3, P=0.024). Importantly, JAK inhibition with ruxolitinib or AZD1480 (1 µM) reversed venetoclax resistance induced by IFNγ, or conditioned media from activated T-cells, or by HS5-CD40L–IL4–IL21 co-culture (n>3, P< 0.05).
Conclusions
We reveal for the first time that IFNγ and IL21 are the key factors driving CLL cell proliferation and survival in lymph nodes. Both activate STAT1, which we identified as responsible for most gene expression changes in LN-resident CLL cells and their ability to proliferate. Additionally, IFNγ mediates robust venetoclax resistance in CLL, which can be effectively reversed by JAK inhibitors (ruxolitinib or AZD1480), providing a rationale for their combination.
Supported by the Ministry of Health of the Czech Republic (grant no. NU22-03-00117 and NW24-03-00369), Czech Science Foundation (grant No.25-15368X), MH CZ-DRO (FNBr, 65269705), MUNI/A/1685/2024.
Keywords : microenvironment, STAT1, venetoclax-resistance
Please indicate how this research was funded. : Supported by the Ministry of Health of the Czech Republic (grant no. NU22-03-00117 and NW24-03-00369), Czech Science Foundation (grant No.25-15368X), MH CZ-DRO (FNBr, 65269705), MUNI/A/1685/2024.
Please indicate the name of the funding organization.: Ministry of Health of the Czech Republic, Czech Health Research Council Czech Science Foundation Masaryk University