Authors
Matthew S. Davids, MD, MMSc, Alen Ostojic, MD, Susana Wargo, MD, Moana Hodari, MS, Jiefen Munley, MD, Richard Hermann, MD.
Introduction
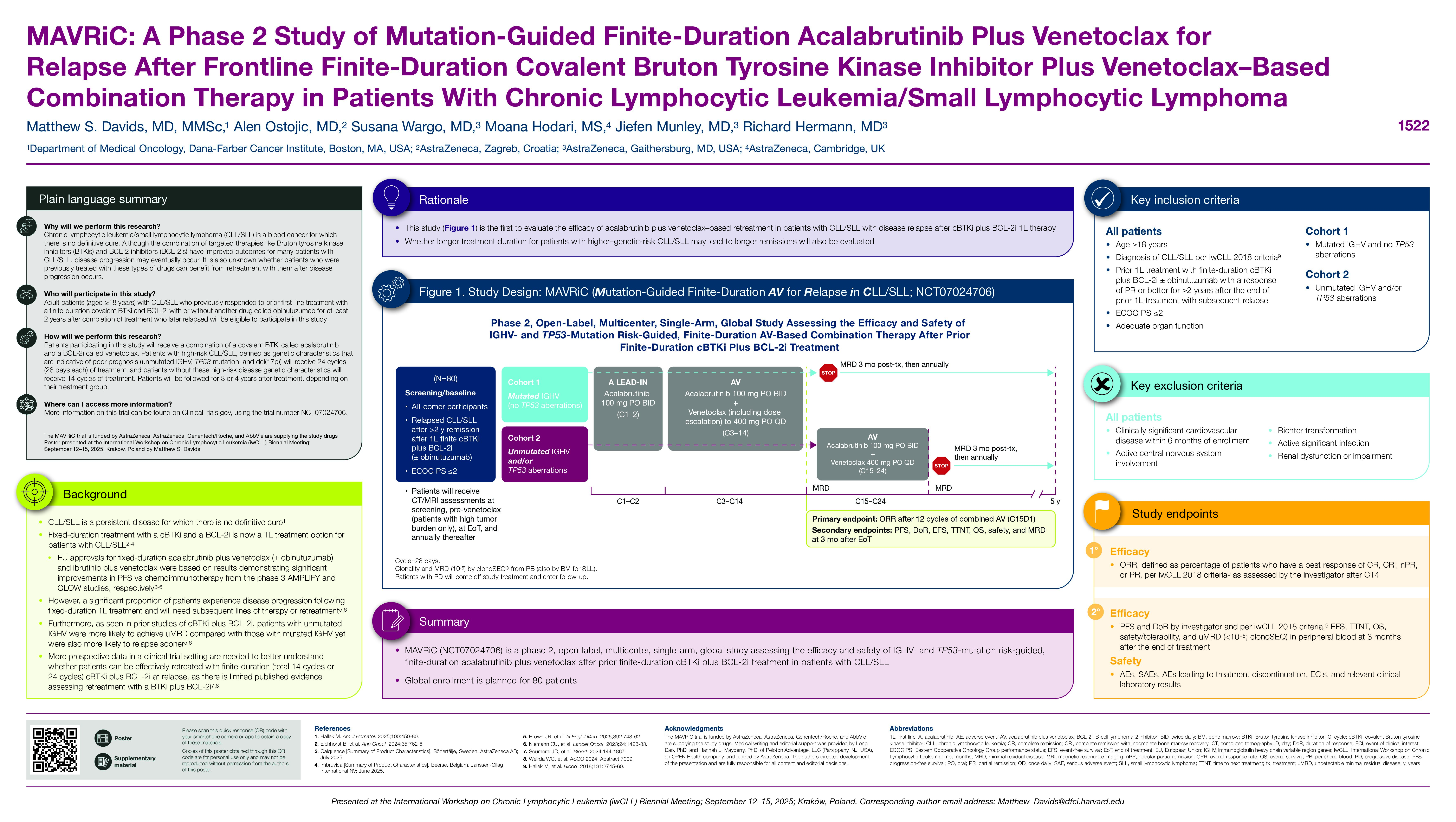
Fixed-duration treatment with a covalent Bruton tyrosine kinase inhibitor (cBTKi) and a B-cell lymphoma 2 inhibitor (BCL-2i) is now a first-line (1L) treatment option for patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). CLL is a persistent disease for which a definitive cure remains elusive. Despite advancements in novel combination targeted therapies, a significant proportion of patients experience disease progression following fixed-duration frontline treatment, necessitating subsequent lines of therapy or retreatment. Furthermore, as seen in prior studies of cBTKi plus BCL-2i, patients with unmutated IGHV were more likely to achieve undetectable minimal residual disease (uMRD) compared with those with mutated IGHV yet were also more likely to relapse sooner. Whether longer treatment duration for patients with higher genetic-risk CLL may lead to longer remissions remains unclear. More prospective data in a clinical trial setting are needed to better understand whether patients can be effectively retreated with finite-duration (total 14 cycles or 24 cycles) cBTKi + BCL-2i at relapse, as the only published data are derived from a small cohort of patients. Fixed-duration (total 14 cycles) combination therapy with the highly selective cBTKi acalabrutinib (A) plus V ± obinutuzumab (O) was found to improve progression-free survival (PFS) compared with chemoimmunotherapy in the phase 3 AMPLIFY trial in patients with TN CLL (Brown JR, et al. NEJM. 2025). The efficacy of AV-based retreatment in patients with CLL who relapse after BTKi + BCL-2i 1L therapy has not been evaluated previously.
Methods
This phase 2, open-label, multicenter, single-arm, global study (MAVRiC: Mutation-guided finite-duration AV for Relapse in CLL/SLL) aims to assess the efficacy and safety of IGHV- and TP53-mutation risk‒guided, finite-duration AV after prior fixed-duration BTKi + BCL-2i treatment. Eligible patients are aged ≥18 y with a diagnosis of CLL/SLL who require treatment according to International Workshop on CLL (iwCLL) 2018 criteria, had prior 1L treatment with finite cBTKi + BCL-2i ± O, and had a partial response or better maintained for ≥2 y after treatment completion with subsequent relapse. Key exclusion criteria include clinically significant cardiovascular disease within 6 months of enrollment, active central nervous system involvement, Richter transformation, active significant infection, and creatinine clearance of < 30 mL/min according to the Cockroft-Gault equation or serum creatinine >2 times the upper limit of normal. Patients will receive AV (A 100 mg oral twice daily [BID] and V 400 mg oral once daily [QD]) in 28-day cycles (C); lead-in A 100 mg BID will be given in C1–2, and V dosing will begin in C3 with the label-prescribed 5-week ramp-up dosing up to 400 mg QD. IGHV and TP53 mutational status will then guide AV duration. Cohort 1 will include patients with lower-risk CLL/SLL (those with mutated IGHV and no TP53 aberrations) treated with AV for C3–14; cohort 2 will include patients with higher-risk CLL/SLL (those with unmutated IGHV and/or TP53 aberrations) treated with AV for C3–24. The primary endpoint is overall response rate per iwCLL 2018 criteria after 12 cycles of combination AV. Key secondary endpoints include PFS, duration of response, event-free survival, time to next treatment, overall survival, safety/tolerability (including tumor lysis syndrome), and rate of undetectable minimal residual disease ( < 10−5) in peripheral blood at 3 months after end of treatment with clonoSEQ. Patients will be followed for 5 years from C1 day 1 until death, withdrawal of consent, or end of study. Global enrollment is planned for 80 patients to begin in 2025 at 25 sites.
Keywords : Chronic lymphocytic leukemia/small lymphocytic lymphoma, Bruton tyrosine kinase inhibitor, retreatment
Please indicate how this research was funded.: Study funded by AstraZeneca
Please indicate the name of the funding organization. : AstraZeneca