Authors
T. Kipps, F. T. Awan, B. Eichhorst, Y. Herishanu, W. Jurczak, D. Lavie, N. Martinez-Calle, A. Masszi, C. Owen, C. Poulsen1, C. Schneider, Y. Xu, J. Yang, M. Z. H. Farooqui, J. Kothari.
Background
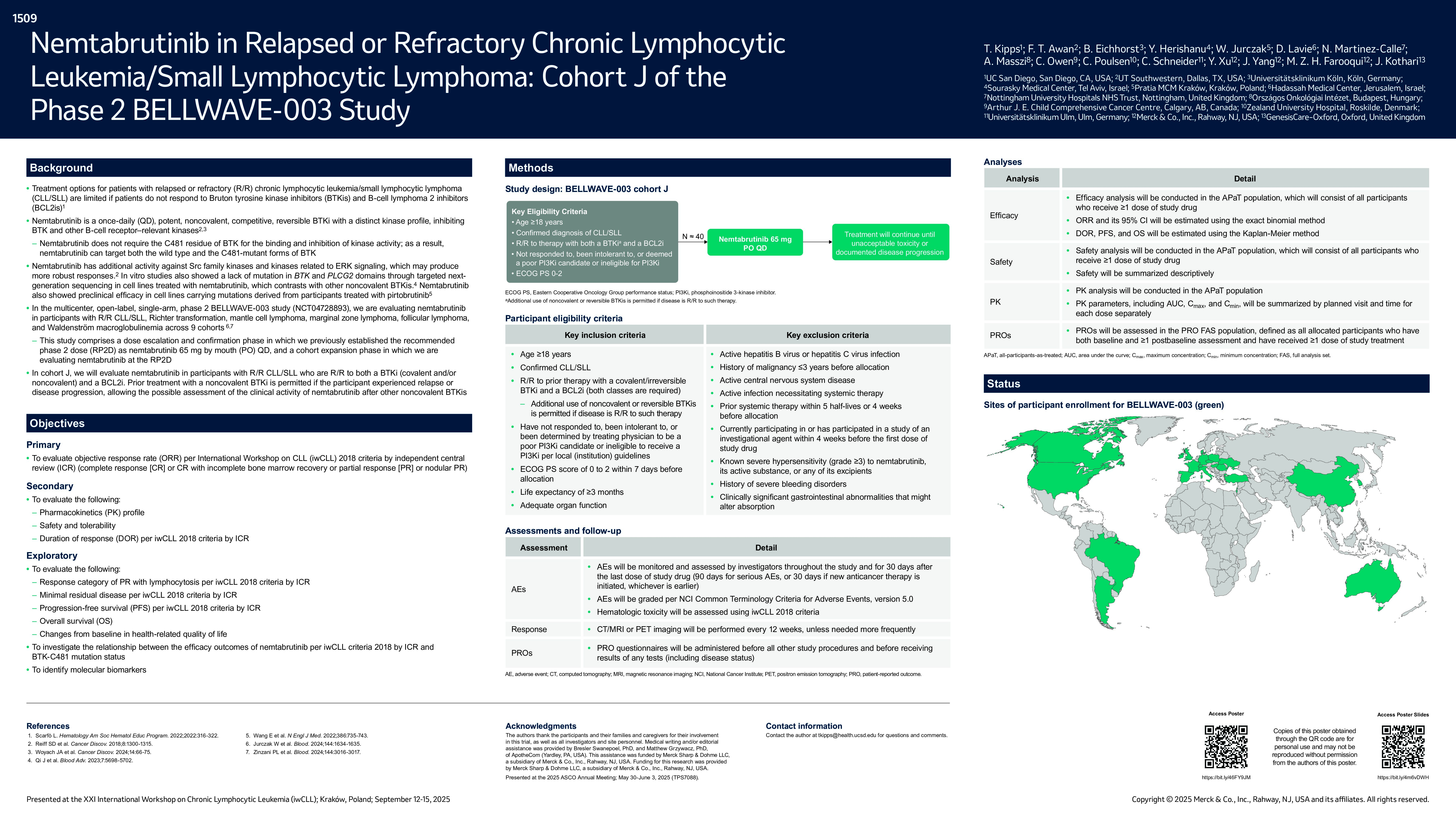
Treatment options are limited for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have relapsed or refractory disease after failing to respond to both Bruton tyrosine kinase (BTK) inhibitors and B-cell lymphoma 2 (BCL2) inhibitors. Nemtabrutinib is a potent, noncovalent, reversible BTK inhibitor with a unique kinase profile that targets and inhibits BTK and other kinases involved in the B-cell receptor pathway. The multicenter, open-label, single-arm, phase 2 BELLWAVE-003 study (NCT04728893) is being conducted to assess nemtabrutinib at the recommended phase 2 dose (RP2D) in participants with relapsed or refractory CLL/SLL, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, Richter transformation, and Waldenström macroglobulinemia. Cohort J specifically focuses on evaluating nemtabrutinib in participants with CLL/SLL who have experienced relapsed or refractory disease to both BTK inhibitor and BCL2 inhibitor treatment.
Methods
Participants eligible for cohort J must be aged ≥18 years with CLL/SLL relapsed or refractory to previous treatment with both a BTK inhibitor (covalent or irreversible) and a BCL2 inhibitor, and have an Eastern Cooperative Oncology Group performance status of 0 to 2. The use of noncovalent or reversible BTK inhibitors is permitted if the disease is relapsed or refractory to such therapy. Participants must have received and not responded to, been intolerant to, or been deemed by their treating physician to be a poor candidate for or ineligible for treatment with phosphoinositide 3–kinase inhibitor per local (institution) guidelines. Exclusion criteria include prior exposure to nemtabrutinib, systemic therapy with a monoclonal antibody within 5 half-lives or 4 weeks before allocation, active central nervous system disease, and clinically significant gastrointestinal abnormalities that might alter absorption. The BELLWAVE-003 study includes a dose escalation and confirmation phase (part 1) to establish the RP2D, followed by a cohort expansion phase (part 2). Part 1 evaluated nemtabrutinib in 6 to 20 participants with relapsed or refractory CLL/SLL after at least 2 prior lines of therapy, establishing the RP2D as 65 mg once daily. In part 2, approximately 460 participants will be enrolled across 9 expansion cohorts with approximately 40 participants in cohort J. Treatment will continue until unacceptable toxicity, disease progression, or participant withdrawal. Adverse events will be monitored and graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0, and hematologic toxicities in participants with CLL will be assessed using International Workshop on Chronic Lymphocytic Leukemia (iwCLL) 2018 criteria. Imaging (CT/MRI and/or PET) will be performed every 12 weeks or as needed. The primary end point for cohort J is objective response rate per iwCLL 2018 criteria by independent central review. Additional end points include overall survival, safety and tolerability, and duration of response and progression-free survival per iwCLL 2018 criteria by independent central review. Recruitment is ongoing, making this the first clinical trial with a dedicated cohort to evaluate noncovalent BTK inhibitors in participants whose disease has not responded to both a BTK inhibitor and BCL2 inhibitor. ©2025 American Society of Clinical Oncology, Inc. Reused with permission. This abstract was accepted and previously presented at the 2025 ASCO Annual Meeting. All rights reserved.
Keywords : Chronic lymphocytic leukemia/small lymphocytic lymphoma, relapsed or refractory, nemtabrutinib
Please indicate how this research was funded.: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
Please indicate the name of the funding organization. : Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA