Authors
Catherine C. Coombs, Jennifer A. Woyach, Jennifer R. Brown, Paolo Ghia, Lindsey E. Roeker, Krish Patel, Toby A. Eyre, Constantine S. Tam, John F. Seymour, Nirav N. Shah, Ian Flinn, Chan Y. Cheah, Shuo Ma, Joanna M. Rhodes, Koji Izutsu, Wojciech Jurczak, William G. Wierda, Lisa M Hess, Naleen Raj Bhandari, Angely Loubert, Paolo B Abada, Nicole Lamanna.
Background
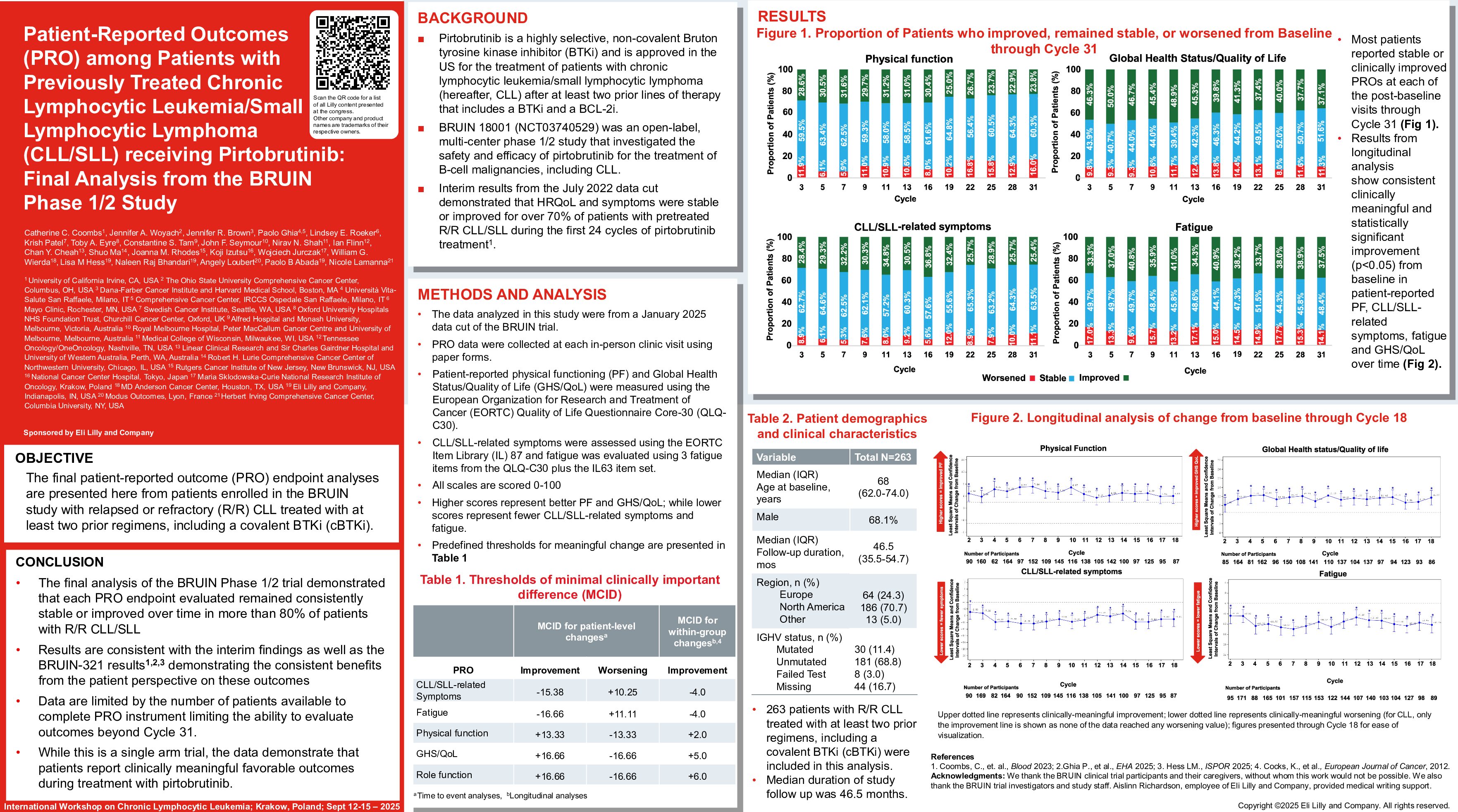
Pirtobrutinib is a highly selective, non-covalent Bruton tyrosine kinase inhibitor (BTKi) and is approved in the US for the treatment of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (hereafter, CLL) after prior systemic therapy that includes a BTKi. BRUIN (NCT03740529) was an open-label, multi-center phase 1/2 study that investigated the safety and efficacy of pirtobrutinib for the treatment of B-cell malignancies, including CLL. The final patient-reported outcome (PRO) endpoint analyses are presented here from patients enrolled in the BRUIN study with relapsed or refractory (R/R) CLL treated with at least two prior regimens, including a covalent BTKi (cBTKi).
Methods
Patients in the BRUIN study completed PRO assessments at each in-person clinic visit using paper forms. Patient-reported physical functioning (PF) and Global Health Status/Quality of Life (GHS/QoL) were measured using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core-30 (QLQ-C30); CLL/SLL-related symptoms and fatigue were assessed using 13 and 6 EORTC Item Library (IL) items, respectively. These were scored from 0-100, where higher scores represent improved PF and GHS/QoL and where higher scores reflect worse CLL/SLL-related symptoms and fatigue. Time to worsening was evaluated using the Kaplan-Meier method for PF, GHS/QoL, CLL/SLL-related symptoms, and fatigue. Events were defined as the first observation of worsening that was sustained through at least one subsequent assessment or patient death, whichever occurred first. Worsening in each PRO endpoint was defined based on pre-specified clinically meaningful within patient change (MWPC) thresholds. Patients without events were censored at their last observed study clinic visit. Pre-specified clinically MWPC thresholds in each PRO score were also used to describe the proportion of patients who improved, remained stable, or worsened based on their individual change from baseline (Cycle 1 Day 1) through Cycle 30 (beyond which fewer patients consistently recorded PRO data). The data cutoff date was 27 January 2025.
Results
There were 263 patients with CLL included in the final analysis with a median (interquartile range) follow-up of 46.5 months (35.5-54.7) from study enrollment. Median age at enrollment was 68 years (62.0-74.0), and the majority were male (68.1%). The completion rate of all PRO measures was 82% at baseline. The baseline mean (± standard deviation) score was 80.8 (±19.5) for PF, 61.6 (±23.2) for GHS/QoL, 24.7 (±18.4) for CLL/SLL-related symptoms, and 33.4 (±24.7) for fatigue. Median time to worsening was not reached for any of the PRO endpoints due to the lack of observed worsening or death events among patients treated with pirtobrutinib. The censoring rates for the time to worsening analyses were high (i.e., 76.9% for PF, 78.1% for GHS/QoL, 78.8% for CLL/SLL-related symptoms, and 73% for fatigue). Most patients reported stable or clinically improved PROs at each of the post-baseline visits through Cycle 30. The proportion of patients who improved or remained stable from baseline through Cycle 30 ranged from 73.2% to 94.1% for PF, 80.4% to 92.0% for GHS/QoL, 86.3% to 94.7% for CLL/SLL-related symptoms, and 78.7% to 91.1% for fatigue.
Conclusion
Consistent with the known favorable safety and efficacy profile of pirtobrutinib, the final analysis of the BRUIN Phase 1/2 trial demonstrated that each PRO endpoint evaluated remained consistently stable or improved over time in more than 70% of patients with R/R CLL/SLL. Data are also limited in that completion of PRO instruments declined over time, limiting the ability to evaluate outcomes beyond Cycle 30. While this is a single arm trial, the data demonstrate that pirtobrutinib provided meaningful benefit to patients during treatment.
Keywords : Patient-reported outcomes, Relapsed/refractory, Pirtobrutinib
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: Loxo Oncology Inc., a wholly owned subsidiary of Eli Lilly and Company