Authors
Tanya Siddiqi MD, John Baird MD, Alexander Boardman MD, Mary Clark PhD, Lu Chen PhD, Vivian Song, Geoffrey Shouse DO, Avyakta Kallam MD, Steven Rosen MD, Alexey Danilov PD PhD, Lili Wang MD PhD.
Rationale
Richter’s transformation (RT) is an aggressive transformation of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) typically to large B cell lymphoma (LBCL) that is difficult to treat and has a poor prognosis. The goal of treatment is to achieve a complete remission (CR) and proceed to consolidative allogeneic hematopoietic cell transplantation (alloHCT). However, outcomes with standard chemoimmunotherapy are dismal, with median OS < 1year (long-term survival < 20%) and CR rates ranging from 5-38%, most of which are transient. Many patients who achieve durable CR are ineligible for alloHCT due to age, comorbidities and/or lack of a suitable donor. Retrospective analyses have shown that chimeric antigen receptor (CAR) T cell monotherapy does not typically yield durable results in RT. Thus, there is an urgent, unmet medical need for well-tolerated, potent therapies for RT. The combination of the BTK inhibitor ibrutinib and the PD-1 checkpoint inhibitor nivolumab has shown activity in RT, with an overall response rate (ORR) of 43% and CR rate of 35%. Most responses to PD-1 inhibition were transient, although potentially could allow bridging to alloHCT. The use of ibrutinib and nivolumab as bridging and maintenance therapy may enhance responses to CAR T cell therapy since CAR T cell products that have PD-1 expression and are skewed toward terminally-differentiated effector T cells are associated with inferior efficacy, suggesting that PD-1 inhibition may enhance CAR T cell efficacy. The safety and approval of lisocabtagene maraleucel (liso-cel) in relapsed/refractory DLBCL as well as CLL makes it the ideal commercial CAR T cell product available in RT where CLL may also be present. We hypothesized that the addition of ibrutinib and nivolumab to CAR T cells in patients with RT may enhance responses to CAR T cell therapy. Therefore, we proposed studying the combination of ibrutinib, nivolumab and liso-cel in patients with relapsed/refractory RT with a curative intent.
Study
Design
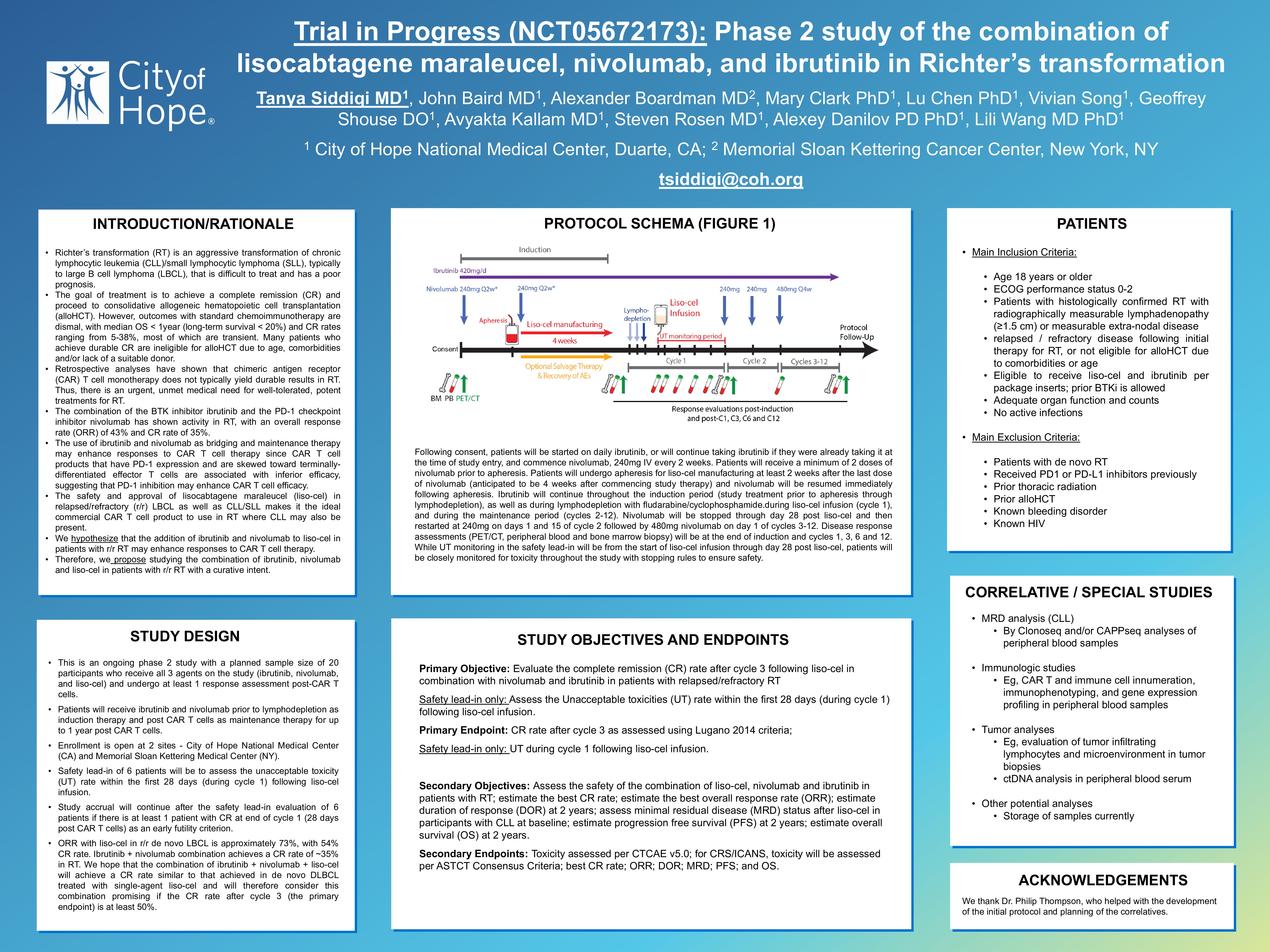
This is a phase 2 study with a planned sample size of 20 participants who receive all 3 agents on the study: ibrutinib, nivolumab, and liso-cel. Enrollment is open at 2 sites – City of Hope National Medical Center (CA) and Memorial Sloan Kettering Medical Center (NY). The primary objective is to evaluate the CR rate after cycle 3 following liso-cel in combination with nivolumab and ibrutinib to treat patients with RT. Safety lead-in of 6 patients will be to assess the unacceptable toxicity (UT) rate within the first 28 days during cycle 1 following liso-cel infusion.
Main Inclusion Criteria
Age 18 years or older; patients with histologically confirmed RT with radiographically measurable lymphadenopathy (≥1.5 cm) or measurable extra-nodal disease; relapsed / refractory following therapy for RT, or not eligible for alloHCT due to comorbidities or age; eligible to receive liso-cel and ibrutinib per package inserts; prior BTKi is allowed; adequate organ function and counts.
Main Exclusion Criteria
Patients with de novo RT; patients who previously received PD1 or PD-L1 inhibitors.
Intervention description
Following consent, patients will be started on daily ibrutinib, or will continue taking ibrutinib if they were already taking it at the time of study entry, and commence nivolumab, 240mg IV every 2 weeks. Patients will receive a minimum of 2 doses of nivolumab prior to apheresis. Patients will undergo apheresis for liso-cel manufacturing at least 2 weeks after the last dose of nivolumab (anticipated to be 4 weeks after commencing study therapy) and nivolumab will be resumed immediately following apheresis. Ibrutinib will continue throughout the induction period (study treatment prior to apheresis through lymphodepletion), as well as during lymphodepletion with fludarabine/cyclophosphamide and liso-cel infusion (cycle 1), and the maintenance period (cycles 2-12). Nivolumab will be stopped through day 28 post liso-cel and then restarted at 240mg on days 1 and 15 of cycle 2 followed by 480mg nivolumab on day 1 of cycles 3-12.
Disease response assessments (PET/CT, peripheral blood and bone marrow biopsy) will be at the end of induction and cycles 1, 3, 6 and 12. While UT monitoring in the safety lead-in will be from the start of liso-cel infusion through day 28 post liso-cel, patients will be closely monitored for toxicity throughout the study with stopping rules to ensure safety.
Keywords : Richter’s transformation, CAR T cell therapy, nivolumab
Please indicate how this research was funded.: Funding and nivolumab provided by Bristol Myers Squibb.
Please indicate the name of the funding organization. : Bristol Myers Squibb.