Authors
Emma Johanne Poulsen Siig, Noomi Vainer, Henrik Hjalgrim, Carsten Utoft Niemann, Christian Brieghel & Emelie Curovic Rotbain.
Introduction
Adverse events (AEs) from treatment of chronic lymphocytic leukemia (CLL) affects quality of life, treatment adherence, and survival. Although targeted therapies have improved prognosis, AEs such as cytopenias and renal impairment remain common. Most evidence on AE risk stems from clinical trials, which often exclude older, frailer and more comorbid patients making findings unrepresentative of routine care populations. We present real-world data on predictors of specific AEs from a nationwide cohort study using comprehensive Danish register data, aiming to support a more personalized and safer approach to CLL treatment.
Methods
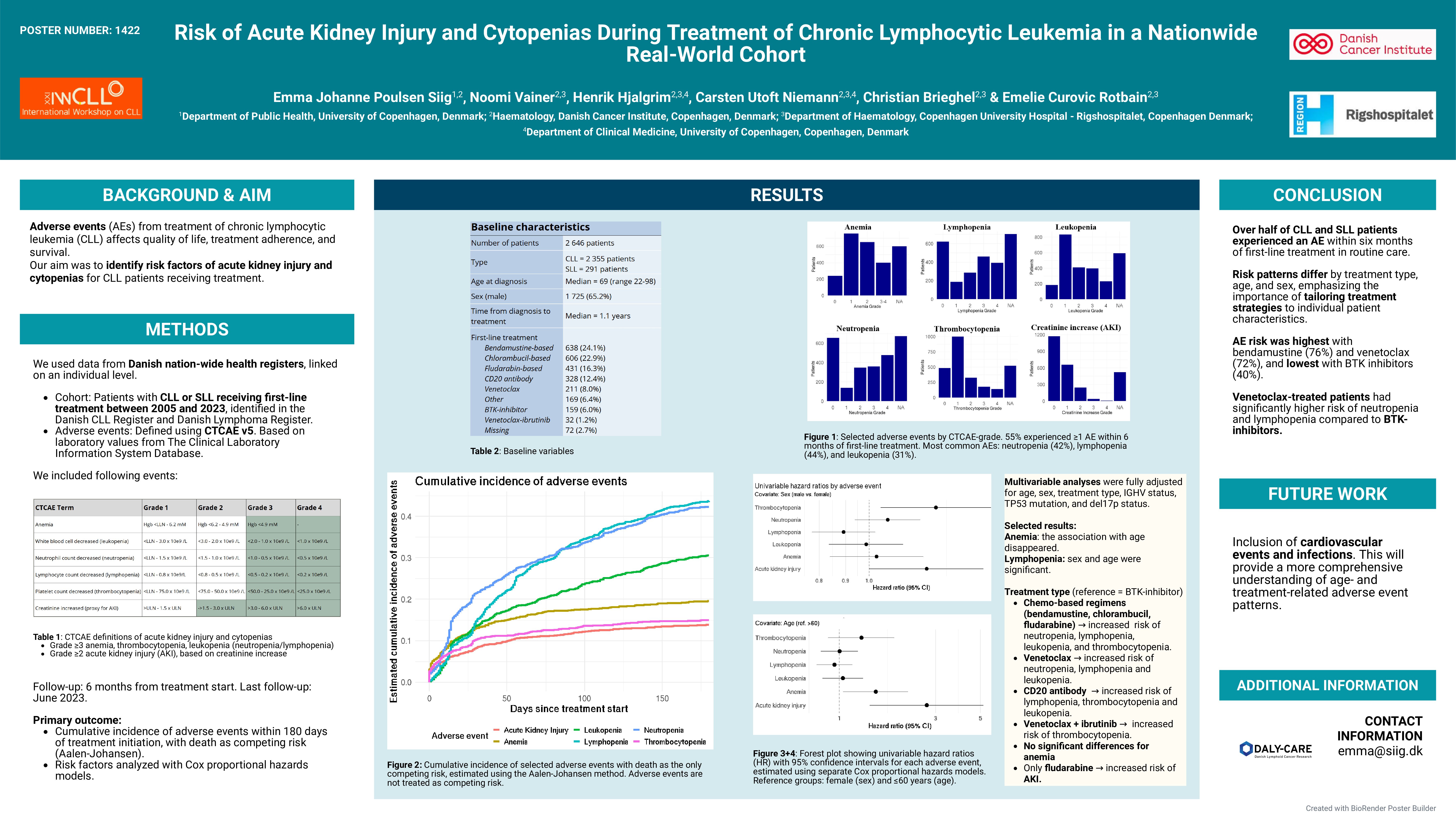
We conducted a nationwide cohort study using the Danish Lymphoid Cancer Research (DALY-CARE) data resource, which integrates national clinical registries, administrative databases, and electronic health records. Patients diagnosed with CLL or small lymphocytic lymphoma (SLL) receiving first-line treatment registered in the Danish National CLL Registry (DCLLR) or the Danish National Lymphoma Registry (LYFO) were included.
AEs were assessed for six months from treatment initiation using Common Terminology Criteria for Adverse Events system version 5. Included AEs were grade ≥3 anemia, thrombocytopenia, leukopenia (neutropenia and lymphopenia), and grade ≥2 acute kidney injury (AKI), using increased creatinine levels as a proxy. Absolute values for laboratory measurements were included without reference to baseline values. Laboratory data were retrieved from the Clinical Laboratory Information System Database.
Descriptive statistics were used to characterize the cohort. Cumulative risk of AEs was estimated using Kaplan-Meier curves, censoring at death (133 patients) or at 180 days of follow-up. Associations between patient characteristics and AEs were analyzed using Cox proportional hazards models.
Results
We included 2,646 patients diagnosed with CLL (n=2,355) or SLL (n=291) between 2005 and 2023, who initiated first-line treatment. 2,531 patients had data on AEs. Median age was 69 years (range 22–98), and 65% were male. Median time from diagnosis to treatment was 1.1 years.
The most common first-line treatment was bendamustine-based (25%), followed by chlorambucil-based (24%), fludarabine-based (17%), CD20 antibody monotherapy (13%) (likely for AIHA/ITP), venetoclax (8%), BTK-inhibitor (6%), and ibrutinib-venetoclax (1%). Other treatments were used in 7%, and 3% had unknown treatment types.
Within six months of treatment initiation, 52% of females and 57% of males experienced at least on AE. AE occurrence varied by age: < 60 years 54%, 60–69 years 58%, 70–79 years 57%, and ≥80 years 49%. The estimated cumulative risk of AE was highest for patients treated with bendamustine (76%) and venetoclax (72%) and lowest with BTK inhibitors (40%). The most common AEs were lymphopenia (44%), neutropenia (42%), and leukopenia (31%), while anemia (20%), thrombocytopenia (15%), and AKI (14%) were less frequent.
In univariable Cox regression analyses, older age was associated with increased risk of anemia, with hazard ratios of 1.51 (95% CI: 1.05–2.19) for patients aged 60–69 years, 1.61 [1.13–2.30] for 70–79 years, and 1.87 [1.29–2.73] for ≥80 years compared with patients < 60 years. Risk of AKI also rose with age (2.71 [1.42–5.17] for 60–69 years, 4.15 [2.24–7.70] for 70–79 years, and 7.82 [4.21–14.55] for ≥80 years). Conversely, age ≥80 years was associated with reduced risk of lymphopenia (0.47 [0.37–0.61]), neutropenia (0.62 [0.49–0.79]), and leukopenia (0.43 [0.32-0.58]). Males had a significantly higher risk of thrombocytopenia (1.35 [1.05–1.73]) and AKI (1.29 [1.00–1.67]) compared with females. No significant sex differences were found for other AEs.
Patients treated with venetoclax alone or in combination with BTK-inhibitors had a higher risk of neutropenia (2.72 [1.84–4.01]), lymphopenia (9.57 [3.85–23.80]) and leukopenia (5.73 [2.73–12.03]) compared to those receiving BTK-inhibitors alone. No significant differences were observed for anemia (p=0.92), thrombocytopenia (p=0.20), or AKI (p=0.06).
Conclusion
Over half of CLL and SLL patients in routine care experienced an AE within six months of first-line treatment. AE risk varied by treatment type, age, and sex. Males had higher risks of thrombocytopenia and AKI. AEs were most frequent with bendamustine or venetoclax, and least frequent with BTK inhibitors and chlorambucil. These findings highlight the importance of patient characteristics when selecting and managing CLL treatment. Future analyses will include cardiovascular events and infections to provide a more comprehensive understanding of age- and treatment-related AE patterns.
Keywords : CLL, adverse events, first-line treatment
Please indicate how this research was funded. : This research did not receive any funding. Co-authors received funding outside this project.
Please indicate the name of the funding organization.: