Targeting Lysosomes in the Chronic Lymphocytic Leukemia Microenvironment (2MB pdf)
Authors
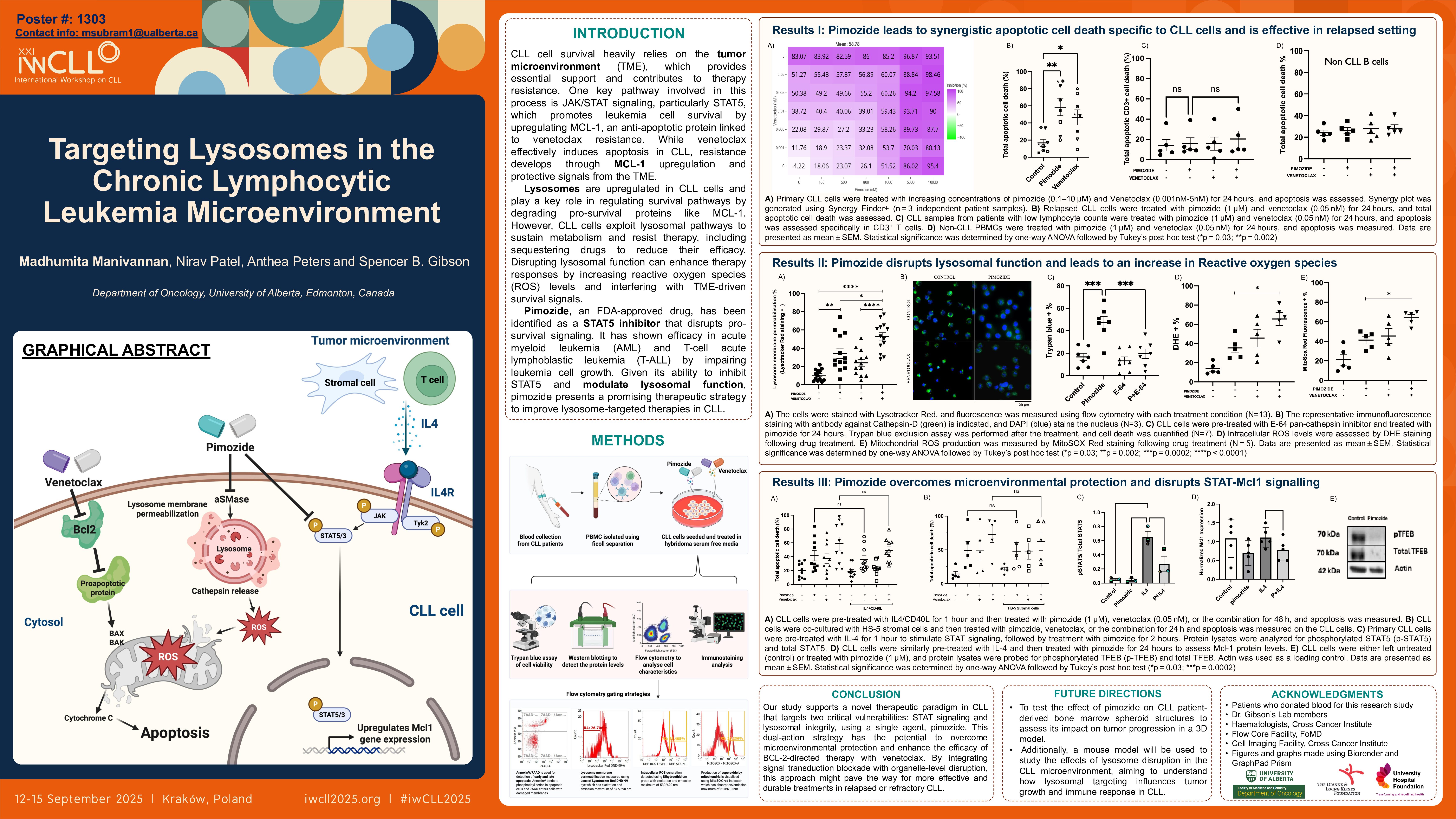
Madhumita Manivannan, Nirav Patel, Anthea Peters and Spencer B. Gibson
Background
Chronic lymphocytic leukemia (CLL) is the most prevalent hematological malignancy and is characterized by the accumulation of monoclonal B cells in the blood, bone marrow, lymph nodes, and spleen (Hallek et al., 2018). Although treatment outcomes have been improved using targeted therapies such as BTK and BCL-2 inhibitors, cures are not achieved, and therapy-resistant disease is frequently observed upon relapse (Lewis et al., 2024). It has been determined that a key driver of drug resistance is the protective microenvironment, which shields CLL cells from apoptosis (Kurtova et al., 2009).
Lysosomes have been identified as emerging therapeutic targets in cancer due to their roles in nutrient recycling, reactive oxygen species (ROS) regulation, and secretory activity through extracellular vesicles (EVs) (Eriksson & Öllinger, 2024; Tang et al., 2020). Lysosome-disrupting agents lead to apoptosis in cancer cells, including CLL cells (Manivannan et al., 2025). Our research identified siramesine as a lysosome-disrupting agent that selectively kills CLL cells by inducing lysosomal membrane permeabilization (LMP), autophagy inhibition, and ROS accumulation (Manivannan et al., 2024). However, since siramesine is not FDA-approved, we repurposed the FDA-approved antipsychotic drug pimozide (Meyer et al., 2017; Nelson et al., 2011).
This study aims to answer whether pimozide can act as an effective lysosome-disrupting agent in CLL and whether it can overcome the protective effects of the microenvironment by inducing lysosomal membrane permeabilization.
Methods
Primary CLL cells were treated with pimozide, and apoptotic cell death was measured using Annexin V/7-AAD staining through a flow cytometer. LMP was assessed via Lysotracker Red staining and confirmed by the cytosolic release of cathepsin D. Intracellular ROS production was detected using DHE, and mitochondrial ROS was detected using MitoSox staining. Autophagy was evaluated through LC3 and LAMP1 co-localization using immunofluorescence microscopy. To mimic the protective effects of the bone marrow microenvironment, we stimulated CLL cells with IL-4/CD40L or co-cultured them with HS-5 stromal cells. Additionally, we analyzed the impact of pimozide on intracellular survival pathways, including STAT3, STAT5, and MCL-1 expression, using Western blotting. Importantly, the cytotoxic effects of these treatments were assessed on non-malignant immune cells and relapsed patient samples using flow cytometry.
Results
Pimozide monotherapy induced pronounced lysosomal membrane permeabilization (LMP), demonstrated by the loss of Lysotracker Red signal and redistribution of cathepsin D into the cytosol. It also impaired the co-localization of autophagosomes with lysosomes, effectively blocking autophagy, a key cell survival mechanism. This lysosomal disruption was associated with elevated levels of intracellular reactive oxygen species (ROS) and mitochondrial ROS. Notably, when combined with venetoclax, a Bcl-2 inhibitor, pimozide markedly enhanced ROS production and mitochondrial superoxide generation, resulting in synergistic induction of apoptotic cell death. Importantly, this combinatorial effect persisted even in the presence of microenvironmental protection conferred by CD40L and IL-4. Pimozide also demonstrated efficacy in primary CLL samples derived from relapsed patients, highlighting its potential in treatment-resistant disease. Importantly, pimozide exhibited selective cytotoxicity toward CLL cells, with minimal impact on normal B cells from healthy donors, as well as CD3⁺ and CD8⁺ T cells.
Mechanistically, pimozide inhibited the phosphorylation of STAT5 and STAT3, key mediators of cytokine-driven survival pathways in CLL, even in the presence of IL-4 stimulation. Additionally, pimozide downregulated the anti-apoptotic protein MCL-1, which is commonly upregulated in venetoclax-resistant CLL, further supporting its potential role in overcoming therapeutic resistance.
Conclusion
The lysosomotropic agent pimozide sensitizes CLL cells to venetoclax by targeting lysosomal integrity, inducing ROS, and inhibiting survival signaling. Pimozide represents a novel treatment strategy for patients with drug-resistant CLL because it induces apoptosis and inhibits cell survival.
Keywords : Lysosome targeted therapy, Drug repurposing, Bone marrow microenvironment
Please indicate how this research was funded. : Lymphatic Chair Establishment Grant: This research grant is associated with an endowed chair in lymphatic disorders. This grant has been awarded to Dr. Spencer B Gibson.
Please indicate the name of the funding organization.: University Hospital Foundation