Authors
Yongyi Yuan, Hao Guo, Siming Zheng, Keshu Zhou.
Introduction
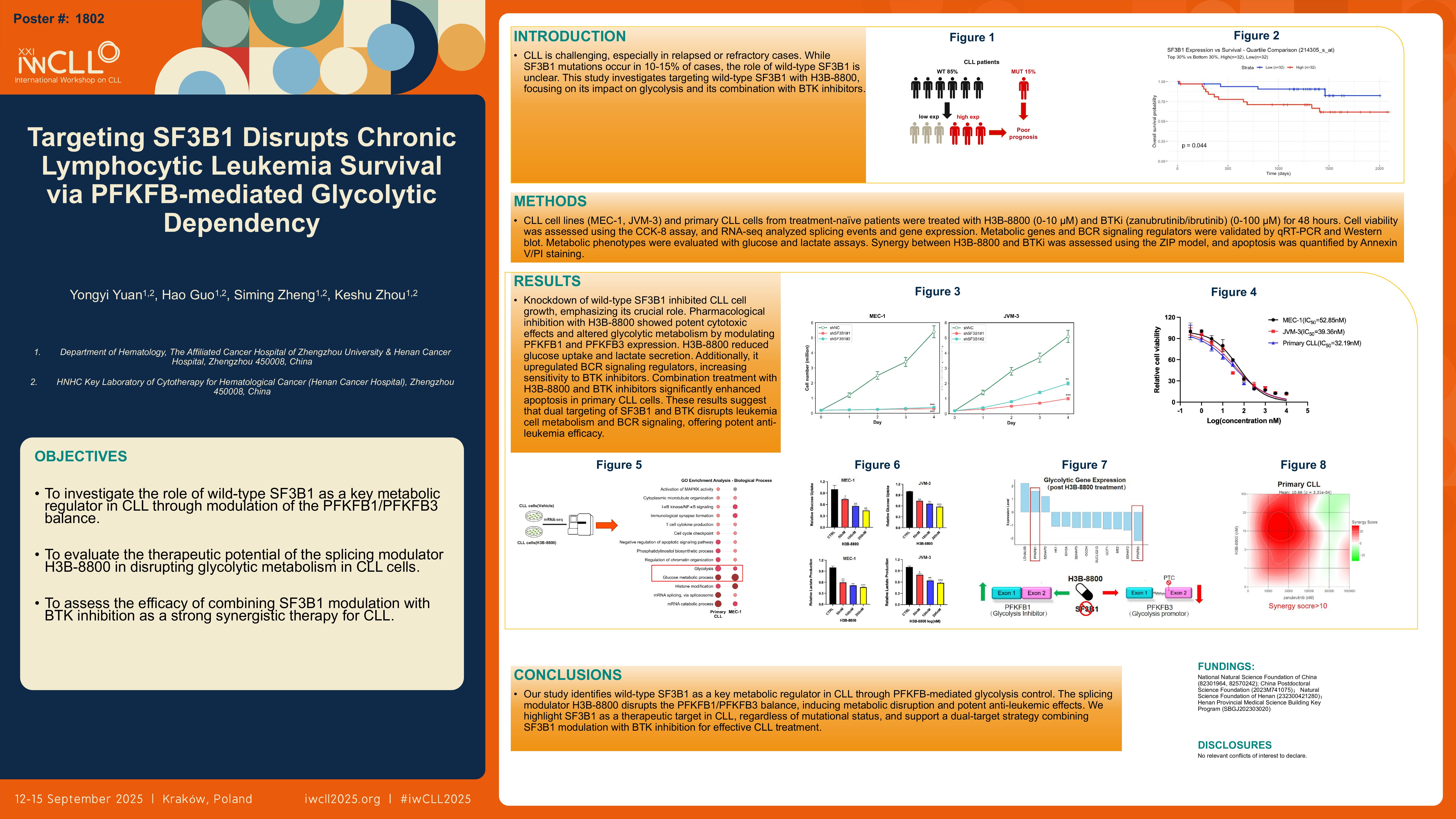
Chronic lymphocytic leukemia (CLL), the most frequent type of leukemia in Western countries, continues to demand novel therapeutic strategies tailored to the subset of patients with rapid progression or relapsed/refractory (R/R) disease. While splicing factor SF3B1 mutations occur in 10–15% of CLL cases, the pathological role of the more prevalent wild-type SF3B1 remains poorly defined. Emerging evidence implicates aberrant RNA splicing in cancer metabolic reprogramming, yet the mechanistic connection between SF3B1-mediated splicing regulation and the metabolic pathways that support CLL cell survival remains unexplored. This study investigates the therapeutic potential of targeting wild-type SF3B1 using the splicing modulator h3b-8800, with a focus on its metabolic consequences in CLL.
Methods
CLL cell lines (MEC-1, JVM3) and primary CD19+ CLL cells from treatment-naïve patients were utilized. Lentiviral shRNA knockdown of SF3B1 in MEC-1 was validated by qRT-PCR. CLL cells were treated with the preclinical SF3B1 modulator h3b-8800 at a gradient of 0–10 μM for 48 hours, and cell viability was assessed using the CCK-8 assay to determine IC50 values. RNA sequencing (RNA-seq) was performed to analyze differential splicing events and gene expression following total RNA extraction. Key metabolic genes were selected and further validated using qRT-PCR and Western blot. Metabolic phenotypes were evaluated using glucose/lactateassay kits.
Results
SF3B1 knockdown significantly inhibited MEC-1 cell growth. h3b-8800 demonstrated potent cytotoxicity across models: IC50 values for 48h were 31.1 nM for primary cells, 32.55 nM for MEC-1, and 68.85 nM for JVM3. Transcriptomic analysis identified an SF3B1 splicing-dependent inverse expression pattern of PFKFB1 (a glycolysis suppressor) and PFKFB3 (a glycolysis activator) upon h3b-8800 treatment. These splicing shifts translated to reciprocal protein expression patterns: PFKFB1 decreased by 78.6% while PFKFB3 increased 186%. Western blot analysis further confirmed the upregulation of PFKFB3 protein expression. Metabolic assays demonstrated that h3b-8800 treatment resulted in a concurrent reduction in glucose uptake(FC=0.793, 20.7% decrease for 50 μM; FC=0.673, 32.7% decrease for 100 μM) and lactate secretion(FC=0.565, 43.5% decrease for 50 μM; FC=0.509, 49.1% decrease for 100 μM).
Conclusions
Our findings establish wild-type SF3B1 as a critical metabolic regulator in CLL through PFKFB-mediated glycolytic control. The splicing modulator h3b-8800 exerts potent anti-leukemic effects by rewiring the PFKFB1/PFKFB3 balance. INotably, our study connects oncogenic spliceosomal dysregulation with metabolic vulnerabilities in CLL, positioning SF3B1-targeting as a promising therapeutic strategy regardless of mutation status. Ongoing investigations are focused on in-depth mechanistic elucidation and efficacy evaluation of the combination of h3b-8800 with first-line targeted therapies .
Keywords : Chronic Lymphocytic Leukemia(CLL); SF3B1; metabolic reprogramming
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: This work was supported by the National Natural Science Foundation of China (82301964), the China Postdoctoral Science Foundation (2023M741075) and the Henan Provincial Medical Science Building Key Program (SBGJ202303020).