Authors
Aseel Alsouqi, Ying Huang, Kerry A. Rogers, Seema Bhat, Michael Grever, Mona Stefanos, Logan Fitzgerald, Michael Keller, Arianna Crouse, Margaret Lucas, Mollie Moran, Cara Grantier, Corinne Hoffman, Mark Reid, Swetha Hinton, Amy S Ruppert, John C. Byrd, Jennifer A Woyach, Kami Maddocks.
Ibrutinib, the first-in-class covalent Bruton tyrosine kinase (BTK) inhibitor, has revolutionized the treatment landscape for CLL/SLL since its approval over a decade ago. Here we report long-term outcomes of ibrutinib in an investigator-initiated trial established prior to its regulatory approval. (NCT01589302).
OSU11133 is a phase II, single-center, open-label, non-randomized trial of ibrutinib monotherapy in patients with relapsed CLL/SLL. Patients were enrolled into two arms depending on presence vs absence of del (17p) by FISH. Patients were eligible if they were ≥18 years with a confirmed diagnosis of CLL/SLL, required treatment per the iwCLL guidelines, and had received ≥ 1 prior therapy. Ibrutinib was given daily at a dose of 420 mg until disease progression or unacceptable toxicity. The primary endpoint was 2-year progression-free survival (PFS). Secondary endpoints included 6-month overall response rate (ORR), overall survival (OS), PFS, and the incidence of adverse events. The Kaplan-Meier method was used to assess PFS and OS. Fine-Gray model treating death without Richter transformation (RT) as the competing risk was used for the univariable analysis. Cox regression models were used to evaluate PFS and OS.
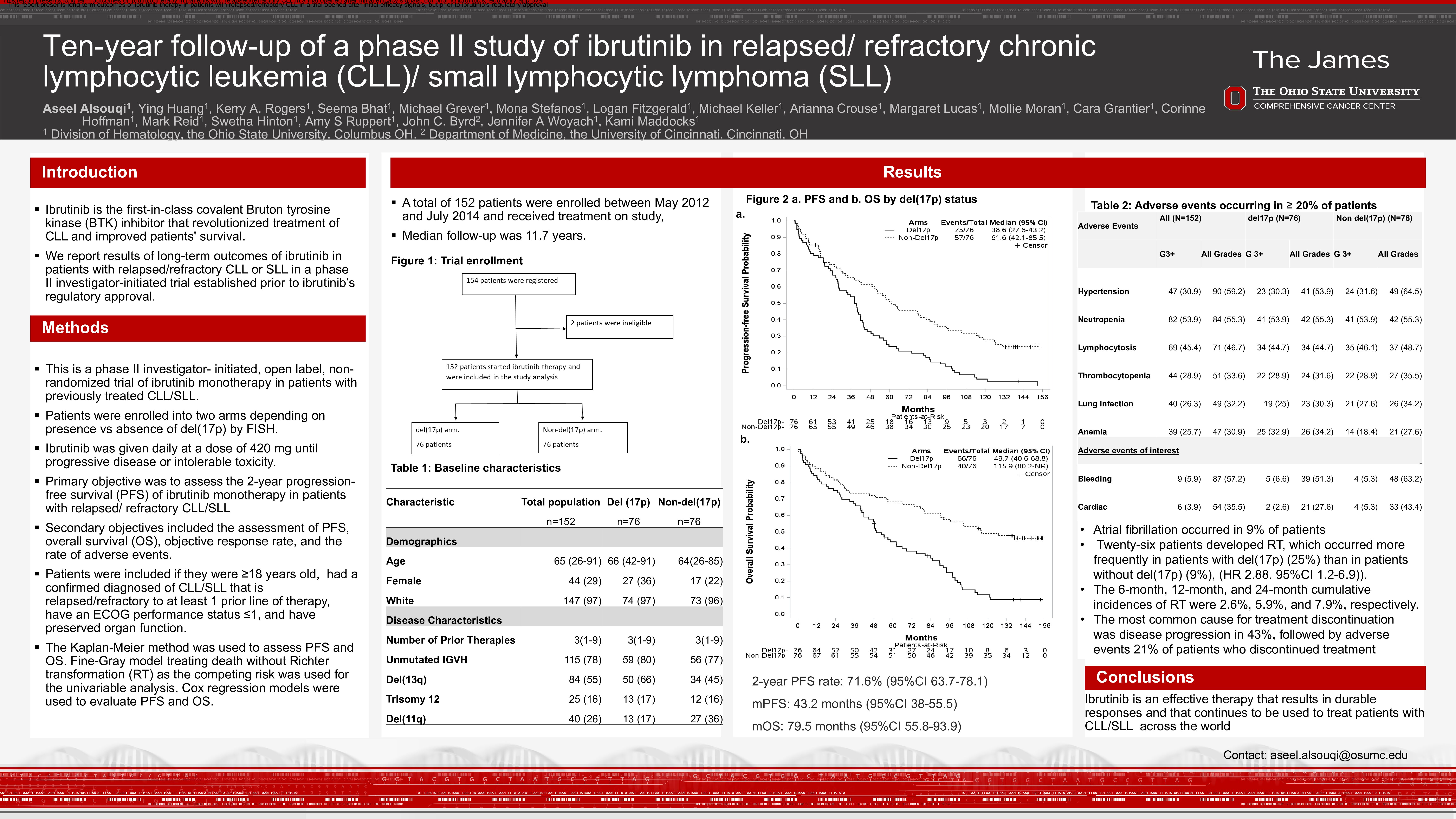
A total of 152 patients were enrolled between May 2012 and July 2014 and received treatment on study, with a median follow-up of 11.7 years. Median age was 65 (range 26-91), 44 (29%) were females, and 147 (97%) were White. Seventy-six patients had del(17p) identified by FISH or karyotype, and 76 did not have del(17p). Among 147 patients with available immunoglobulin variable region heavy chain (IGHV) mutational status, 115 (78%) patients had unmutated IGHV and 32 (22%) had mutated IGHV. Del(11q) was identified in 40 patients including 13 patients in the del(17p) arm. The median number of prior therapies was 3 (range 1-9). The 2-year PFS rate was 71.6% (95%CI 63.7-78.1) and the median PFS was 43.2 months (95%CI 38-55.5). Among patients with and without del(17p), median PFS was 38.6 (95%CI 27.6-43.2) and 61.6 months (95%CI 42.1-85.5), respectively. Multivariable models demonstrated that del(17p), higher number of prior therapies, high lactate dehydrogenase (LDH) at baseline, and IGHV unmutated status were associated with shorter PFS. The median OS was 79.5 months (95%CI 55.8-93.9). Median OS for patients with and without del(17p) was 49.7 months (95%CI 40.8-68.6) and 115.9 months (95%CI 80.2- not reached), respectively. Multivariable models demonstrated del(17p), number of prior therapy lines, and higher LDH at baseline to be associated with shorter OS, while higher hemoglobin at baseline was associated with improved OS. When including partial response with lymphocytosis, the ORR was 81.7%, regardless of del(17p) status. Twenty-six patients developed RT, which occurred more frequently in patients with del(17p) (19 patients, 25%) than in patients without del(17p) (7 patients, 9%), hazard ratio 2.88 (95%CI 1.2-6.9)). The 6-month, 12-month, and 24-month cumulative incidences of RT were 2.6%, 5.9%, and 7.9%, respectively. Univariable models demonstrated del(17p), female sex, and higher LDH at baseline to be associated with higher risk of RT.
Out of 93 patients tested routinely for BTK mutations during treatment on the study, BTK mutations were detected in 53 (57%) patients: 27 (out of 48) with del(17p) and 26 (out of 45) without del(17p). PLCG2 mutations were detected in 8 patients: 7 with del(17p) and 1 without del (17p). Four patients remain on treatment on trial, with a median of 12 years of treatment.
Grade 3 or higher treatment emergent adverse events occurring in >20% of patients included neutropenia (53.9%), hypertension (30.9%), thrombocytopenia (28.9%), pneumonia (26.3%), and anemia (25.7%). Atrial fibrillation occurred in 9% of patients.
This report presents long term outcomes of ibrutinib therapy in patients with relapsed/refractory CLL in a trial opened after initial efficacy signals, but prior to ibrutinib’s regulatory approval. While newer generation BTK inhibitors have demonstrated more favorable toxicity profiles, ibrutinib remains an effective therapy that results in durable responses and that continues to be used across the world. The higher incidence of RT observed is likely related to the higher number of patients with adverse risk characteristics and prior chemotherapy treatments. We emphasize the importance of investigator-initiated trials that are inclusive of high risk disease populations, and that provide life-saving therapies and valuable insight into long-term outcomes and safety.
Keywords : CLL, relapsed/ refractory, ibrutinib
Please indicate how this research was funded. : Investigator initiated trial
Please indicate the name of the funding organization.: