The CDK2/9 inhibitor fadraciclib in Richter transformation (1MB pdf)
Authors
Yuling Chen, Ping Xiong, William Wierda, William Plunkett and Rong Chen
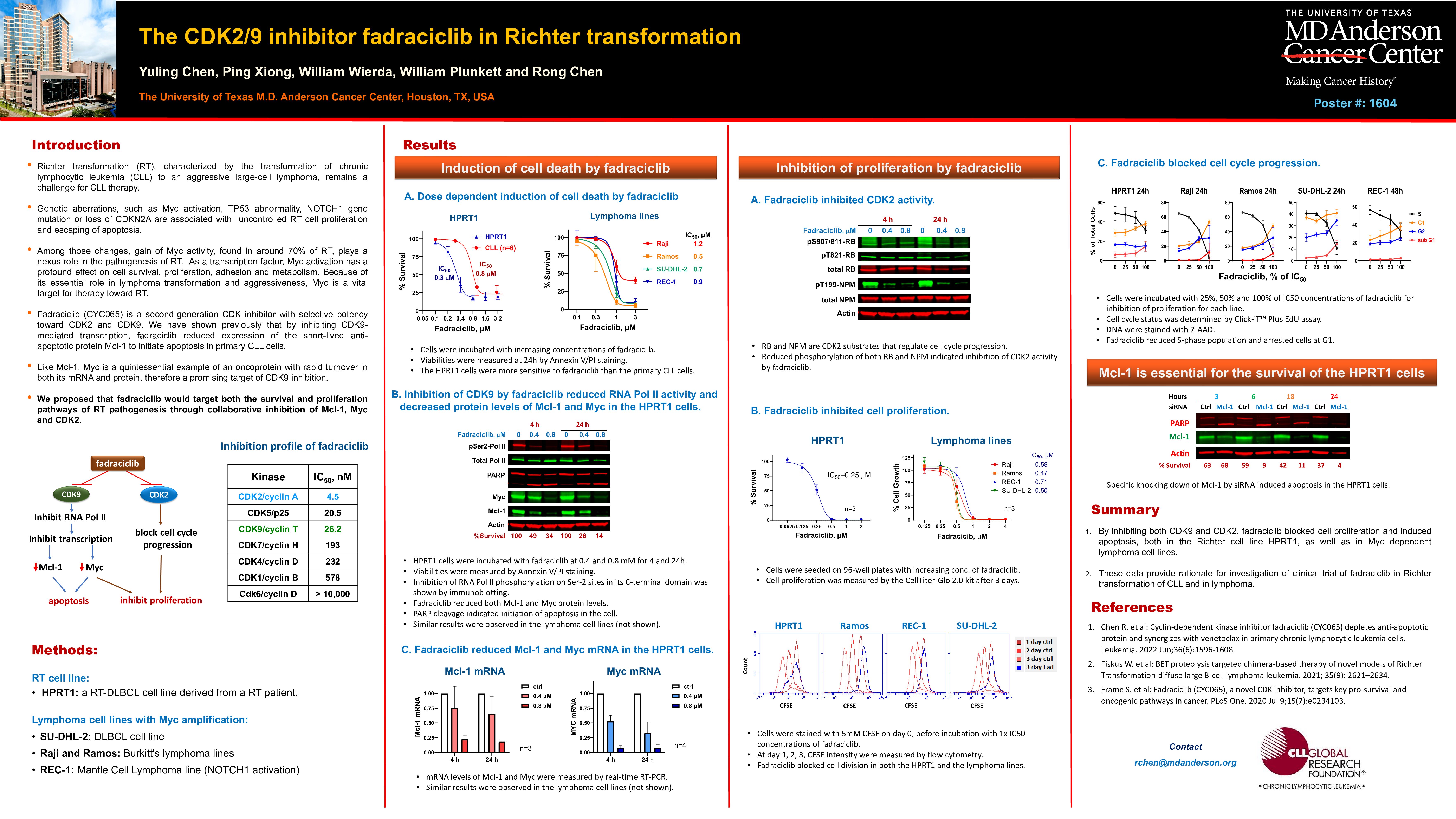
Richter transformation (RT)—the progression of chronic lymphocytic leukemia (CLL) into an aggressive large-cell lymphoma—remains a significant therapeutic challenge. This transformation is frequently driven by genetic alterations such as MYC activation, TP53 abnormalities, NOTCH1 mutations, and CDKN2A loss, which collectively promote uncontrolled proliferation and evasion of apoptosis. Among these, Myc activation—detected in approximately 70% of RT cases—plays a central role in RT pathogenesis. As a transcription factor, Myc exerts broad effects on cell survival, proliferation, adhesion, and metabolism, making it a compelling therapeutic target in RT.
Fadraciclib (CYC065) is a second-generation cyclin-dependent kinase (CDK) inhibitor with selectivity for both CDK2 and CDK9. We previously demonstrated that fadraciclib induces apoptosis in primary CLL cells by inhibiting CDK9-mediated transcription, leading to downregulation of the short-lived anti-apoptotic protein Mcl-1. Similar to Mcl-1, Myc is a rapidly turned-over oncoprotein at both the mRNA and protein levels, making it an attractive target for CDK9 inhibition. Combined with the essential role of CDK2 in cell cycle progression, we hypothesized that fadraciclib could simultaneously target key survival and proliferative pathways in RT through coordinated inhibition of Mcl-1, Myc, and CDK2.
Our findings support this hypothesis through three key observations:
First, CDK9 inhibition was evident in the RT cell line HPRT1 and in MYC-amplified lymphoma cell lines (Raji, Ramos, REC-1 and SU-DHL-2), as demonstrated by reduced phosphorylation of RNA polymerase II (RNA Pol II), indicative of decreased transcriptional activity. This was accompanied by marked reductions in both mRNA and protein levels of Mcl-1 and Myc, confirmed by real-time RT-PCR and immunoblotting. CDK2 inhibition was also observed, as shown by decreased phosphorylation of its downstream targets, RB and NPM. Notably, siRNA-mediated knockdown of Mcl-1 induced apoptosis in HPRT1 cells within three hours, underscoring the cells’ critical dependence on Mcl-1 for survival.
Second, fadraciclib effectively induced apoptosis in these cell lines. In HPRT1 cells, the IC₅₀ for apoptosis was 0.3 µM at 24 hours—substantially lower than the average IC₅₀ in primary CLL cells (0.8 µM). In the MYC-amplified lymphoma cell lines, IC₅₀ values for apoptosis ranged from 0.8 to 1.3 µM.
Third, fadraciclib suppressed cell proliferation. In HPRT1 cells, the IC₅₀ for growth inhibition was 0.25 µM, as measured by CellTiter-Glo analysis. In the lymphoma lines, IC₅₀ values ranged from 0.5 to 0.7 µM. CFSE tracking confirmed reduced cell division and Click-iT EdU incorporation assays showed decreased S-phase entry and cell cycle arrest at the G1 phase, consistent with CDK2’s role in G1/S transition.
In summary, our results demonstrate that fadraciclib is active in RT and Myc-driven lymphoma cell lines by targeting both survival and proliferation pathways, supporting its further clinical evaluation in patients with Richter transformation.
Keywords : Fadraciclib, Richter transformation, CDK
Please indicate how this research was funded. : The research was funded by a grant from the CLL Global Research Foundation
Please indicate the name of the funding organization.: CLL Global Research Foundation