The impact of body mass index on overall survival in chronic lymphocytic leukemia (7MB pdf)
Authors
Noomi Vainer, Henrik Hjalgrim, Klaus Rostgaard, Carsten U. Niemann, Emelie C. Rotbain.
Background
Comorbidities are common in patients with chronic lymphocytic leukemia (CLL) due to the typical high age at diagnosis. Cardiometabolic conditions such as hypertension and type 2 diabetes (T2D), among the most common comorbidities in patients with CLL (prevalence 48% and 12% respectively), are associated with lower overall survival. Being overweight/obese is closely intertwined with the development of cardiometabolic disease. Little is known about whether the impact of the comorbidities is due to the presence of the diseases or inherent in the risk factors associated with being overweight.
Aim
To assess the impact of body mass index (BMI) on treatment and survival outcomes for patients with CLL from time of CLL treatment.
Methods
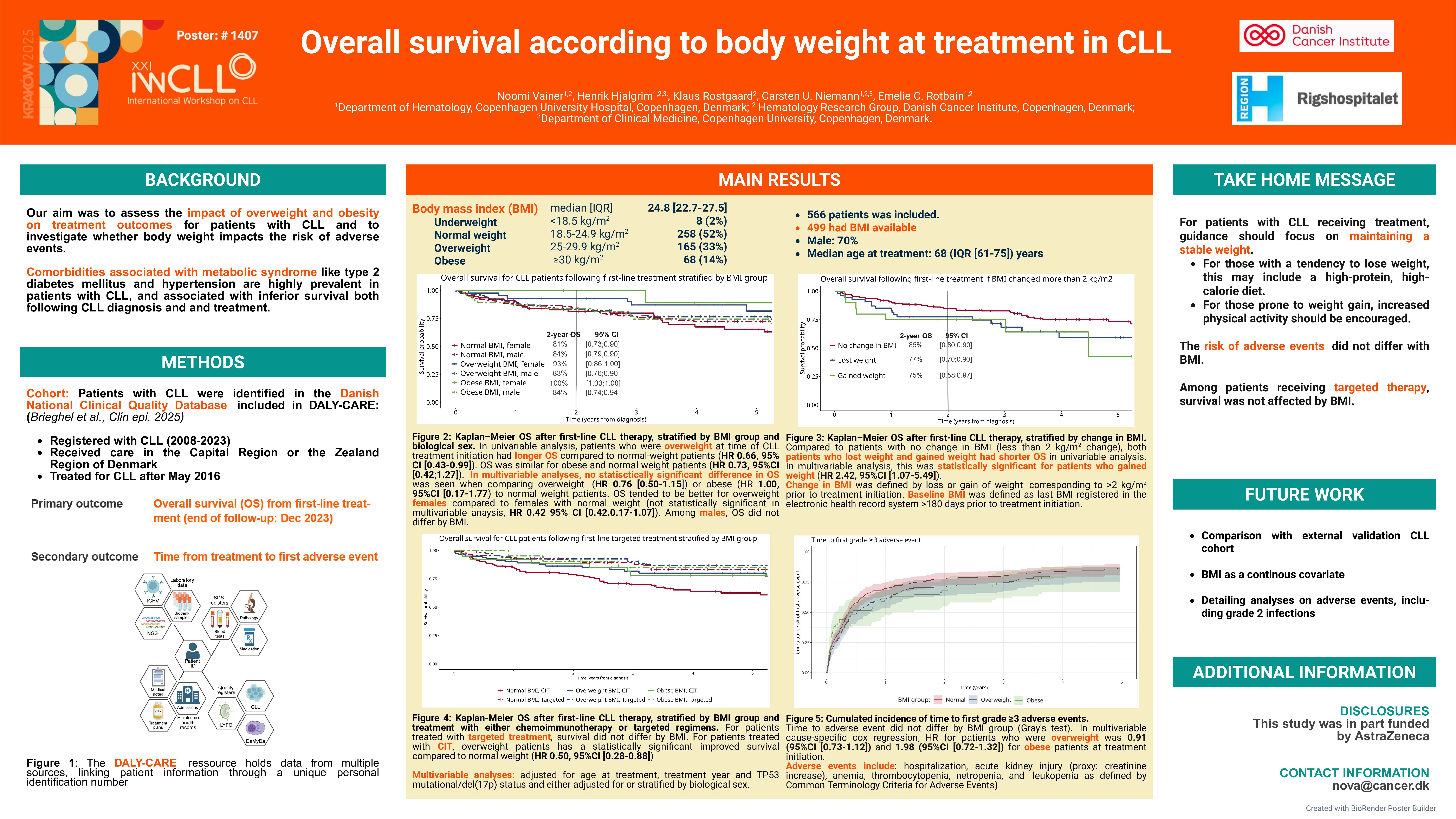
The DALY-CARE data source holds data from the Danish National Clinical Quality Database for CLL, in which we identified patients registered from 2008 to 2023 receiving care in the Capital Region and the Zealand Region of Denmark and treated for CLL after May 2016. Patients were followed from first-line treatment until death or end of follow-up, which was 31st of December 2023. Event analysis following first line treatment were conducted using Kaplan-Meier estimates and Cox’s proportional hazard model adjusted for age at first-line CLL treatment, sex, treatment year and presence of TP53 mutation/aberration or deletion 17p.
Results
We identified 566 patients of whom 499 patients with information on BMI available at time of first line treatment (88%) were included in further analysis; 70% were male and the median age at treatment initiation was 68 years (IQR 61-75). Univariable Cox regression showed better event-free survival (EFS) and overall survival (OS) for overweight patients with CLL compared to normal weight patients with CLL (hazard ratio (HR): 0.66, 95% confidence interval (CI) [0.46;0.95] and HR: 0.63, 95% CI [0.42;0.96], respectively). No difference in risk was found for EFS or OS in obese (p=0.27 and p=0.26) compared to normal BMI patients. Female obese and overweight patients had better survival compared to their male comparators within BMI groups, while no difference between sex was seen for those with normal BMI (Figure 1). For patients receiving targeted treatment, no difference was seen between BMI groups for EFS (p=0.74) or OS (p=0.62) in univariate analysis. In multivariable analysis adjusted for sex, age at treatment, treatment year and presence of TP53 mutation/aberration or deletion 17p, overweight patients had decreased EFS (HR: 0.73 95% CI [0.51;1.05]) and OS (HR: 0.75 95% CI [0.50;1.14]) compared with normal weight patients, although not statistically significant. No difference was seen in multivariable analysis between obese and normal weight patients for either EFS (p=0.97) or OS (p=0.97). In multivariable analysis, obese patients had an increased OS (HR: 1.33 95% CI [0.72;2.46]) and EFS (HR: 1.54 95% CI [0.94;2.55]), although not statistically significant. We did not have enough events in the underweight BMI group to report any meaningful risk for this group. To secure simple interpretation of results, underweight people were therefore excluded from the analyses.
Conclusion and further analysis
These preliminary findings on the association between BMI and survival from first-line treatment in CLL, suggest that obesity may not be a risk factor in CLL, and that being overweight might even be protective. This highlights the importance of nutritional optimization in patients treated for CLL. To further inform management of CLL, these results will be followed up by assessing patterns of toxicity in fixed-dosage chemoimmunotherapy and targeted treatment, comparing real-world data with a clinical trial cohort while also including genetic risk for increased BMI.
Keywords : Chronic lymphocytic leukemia, BMI, survival
Please indicate how this research was funded. : Noomi Vainer received funding for her PhD from AstraZeneca. The funding source had no influence on the decision to submit this abstract, the final content of this abstract, or the interpretation of the data.
Please indicate the name of the funding organization.: This research was supported in part by AstraZeneca