Authors
Abhishek Pethe, Julius Plomer, Lixia Li, Danielle-Justine Danner, Andrea Härzschel, Peter Krenn, Claas Tapken, Adrián Fernández-Rego, Laura Polcik, Driti Ashok, Melissa Riedle, Sandra Kissel, Cornelius Miething, Justus Duyster, Natalie Köhler, Palash C. Maity, Elias Hobeika, Yolanda Carrasco, Tanja Nicole Hartmann.
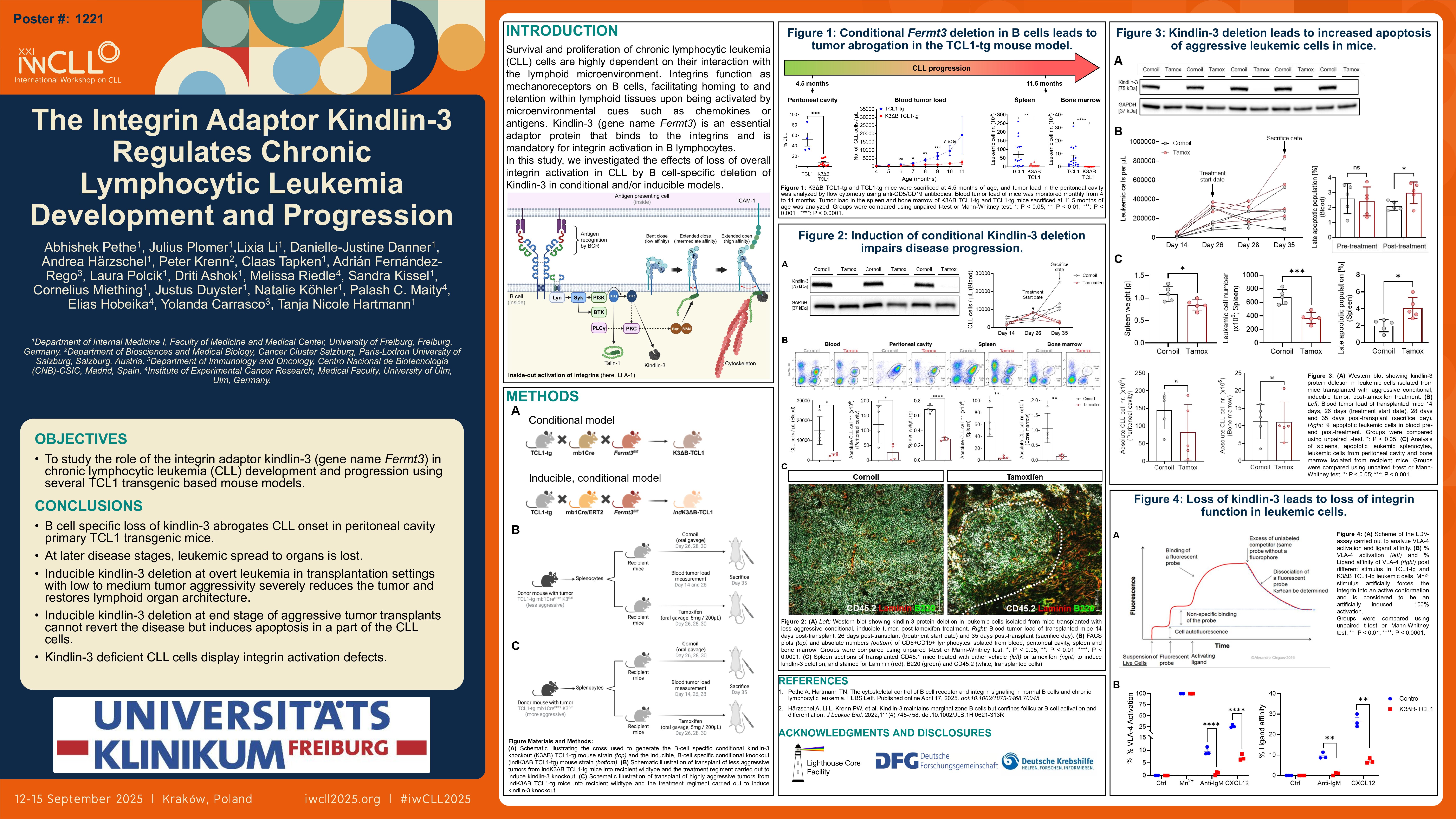
Chronic lymphocytic leukemia (CLL) is a CD5+CD19+ B cell malignancy of the elderly. Tumor proliferation is driven by interactions of CLL cells with the microenvironment in secondary lymphoid organs. Integrins, stabilized by adaptor proteins such as kindlin-3, mediate these interactions and carry out functions such as modulation of B cell receptor clustering and antigen-triggered signaling. Previous research in the lab showed that B cell specific deletion of kindlin-3 in mice (hereafter referred to as K3∆B) promotes antigen-independent B cell activation and differentiation, and hyperactivates CXCR5 signaling. Here, we investigated the role of kindlin-3 in development and progression of CLL.
We generated a B cell specific kindlin-3 knockout mouse model of CLL-like disease, by crossing K3∆B mice with the Eµ-TCL1-tg mouse model of CLL (here on referred to as TCL1-K3∆B). Early time point analysis revealed that kindlin-3 deficiency almost completely abolished tumor onset in the peritoneal cavity. Blood tumor loads were significantly lesser in TCL1-K3∆B mice through development, and end-point analysis showed that tumors were almost completely abrogated in the spleen and bone marrow, compared to TCL1-tg littermates. Using an inducible kindlin-3 knockout TCL1-tg model and transplantation experiments, we observed that kindlin-3 deficiency, in the short-term, leads to altered CLL cell localization in the spleen. Long-term engraftment experiments revealed that kindlin-3 deficient tumor cells displayed significantly lower levels of the negative prognostic markers CD49d (the alpha subunit of the integrin VLA-4) as well as CXCR4. Importantly, in these mice, an induced knockout of kindlin-3 led to reduction in blood tumor loads and arrested tumor progression.
In summary, our research unveils an important role of kindlin-3 in CLL pathogenesis, playing an indispensable role in CLL onset as well as progression.
Keywords : chronic lymphocytic leukemia, integrin adaptor, tumor microenvironment
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: