Authors
S. Gonçalves, J. Jorge, R. Gomes, R. Alves, A. C. Gonçalves, J.P. Carda, A. B. Sarmento-Ribeiro, S. Duarte.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by proliferation and accumulation of monoclonal B lymphocytes, of which pathophysiology involves chromosomal abnormalities, somatic mutations, and dysregulated signalling pathways, including B-cell receptor (BCR) and BCL2-mediated apoptosis resistance. Oxidative stress (OS) plays a crucial role in CLL development, progression and resistance to therapy. Nuclear factor erythroid 2-related factor 2 (NRF2), a major oxidative stress regulator, is deregulated in CLL cells. Current treatment strategies targeting BCR and BCL2 are associated with prolonged progression free survival. However, resistance mechanisms, particularly mutations in Bruton tyrosine kinase (BTK) and in BCL2, pose significant therapeutic challenges. Novel strategies, such as NRF2 modulation with Brusatol (BRU), an enhancer of ubiquitination and degradation of NRF2, may offer alternative therapeutic approaches. This study evaluates the impact of NRF2 pathway inhibition in CLL patient’s cells using Brusatol in monotherapy and in combination with conventional therapy, namely ibrutinib and/or venetoclax.
Methods
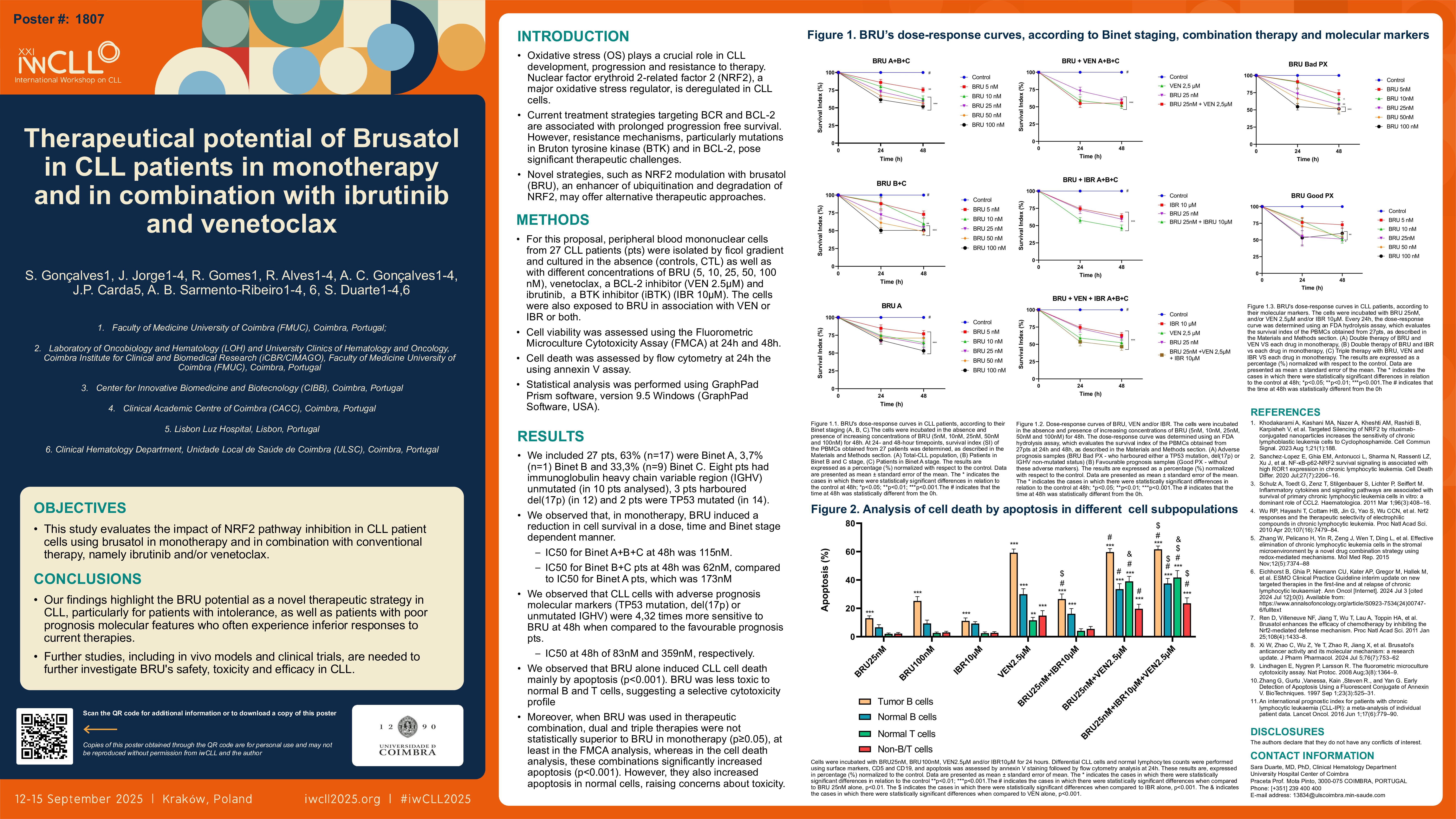
For this proposal, peripheral blood mononuclear cells from 27 CLL patients (pts) were isolated by ficol gradient and cultured in the absence (controls, CTL) as well as with different concentrations of BRU (5, 10, 25, 50, 100 nM), Venetoclax, a BCL-2 inhibitor (VEN 2.5uM) and Ibrutinib, a BTK inhibitor (iBTK) (IBR 10uM). The cells were also exposed to BRU in association with VEN or IBR or both. Cell viability was assessed using the Fluorometric Microculture Cytotoxicity Assay (FMCA) at 24h and 48h. Cell death was assessed vy flow cytometry at 24h the using Annexin V assay. Statistical analysis was performed using GraphPad Prism software, version 9.5 Windows (GraphPad Software, USA).
Results
We included 27 pts, 74% male, mean age at diagnosis of 69.1 years. At the time of analysis, 63% (n=17) of pts were Binet A, 3,7% (n=1) Binet B and 33,3% (n=9) Binet C. Eight pts had immunoglobulin heavy chain variable region (IGHV) unmutated (in 10 pts analysed), 3 pts harboured del17p (in 12) and 2 pts were TP53 mutated (in 14).
We observed that, in monotherapy, BRU induced a reduction in cell survival in a dose, time and Binet stage dependent manner. IC50 at 48h was 115nM. We also observed a bigger therapeutic effect with lower drug dose in CLL cells harbouring either a TP53 mutation, del17p, and/or a unmutated IGHV when compared to those who did not have these molecular characteristics, as in first cases the IC50 at 48h is lower, about 83nM, compared with those we didn´t have these characteristics (359nM).
Further, we observed that BRU alone induced CLL cell death mainly by apoptosis (p < 0.001).
Moreover, when BRU were used in therapeutic combination, dual and triple therapies were not statistically superior to BRU in monotherapy (p≥0.05), at least in the FMCA analysis, whereas in the cell death analysis, these combinations significantly increased apoptosis (p < 0.001). However, they also increased apoptosis in normal cells, raising concerns about toxicity.
Conclusion
Our results suggest an effect of NRF2 inhibition on CLL cell survival, highlighting BRU as a potential therapeutic armamentum in CLL pts, potentially in those who have contraindication or resistance to BTKi or VEN. These findings support NRF2 as a novel therapeutic target in CLL, however further investigation into combination strategies and its role for overcoming treatment resistance are needed.
Keywords : “Brusatol”, “ibrutinib”, “venetoclax”
Please indicate how this research was funded.:
Please indicate the name of the funding organization. :