Authors
Emma Kennedy, Lauren Stott, Iona Ashworth, Rosalynd Johnston, John Jones, Eleni Ladikou, Valter Gattei, Antonella Zucchetto, Fabio Simoes, Simon Mitchell, Chris Pepper, Andrea Pepper.
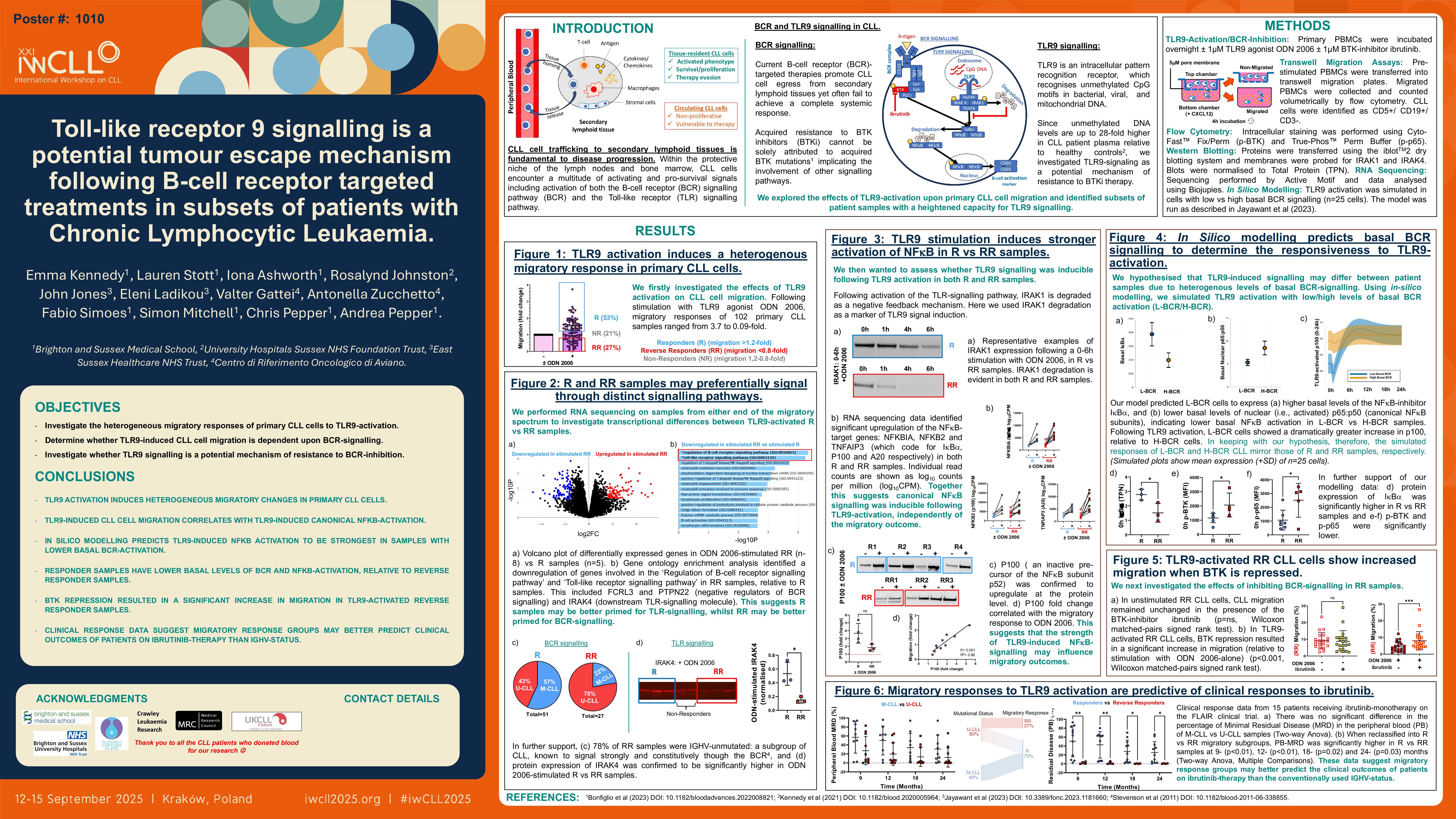
Background
Current B-cell receptor (BCR)-targeted therapies promote CLL cell egress from secondary lymphoid tissues yet often fail to achieve a complete systemic response. Acquired resistance to BTK inhibitors (BTKi) cannot be solely attributed to acquired BTK mutations (Bonfiglio et al., 2023), implicating the involvement of other signaling pathways. Toll-like receptor 9 (TLR9) is an intracellular pattern recognition receptor, which recognizes unmethylated CpG motifs in bacterial/viral/mitochondrial DNA. Since unmethylated DNA levels are up to 28-fold higher in CLL patient plasma relative to healthy controls (Kennedy et al., 2021), we investigated TLR9-signaling as a potential mechanism of resistance to BTKi therapy. We explored the effects of TLR9-activation upon primary CLL cell migration and identified subsets of patient samples with a heightened capacity for TLR9 signaling.
Methods
Primary PBMCs were stimulated overnight ± ODN 2006 (TLR9 agonist) ± ibrutinib (BTKi) ± ODN INH-18 (TLR9 antagonist) and transferred into transwell migration chambers to migrate against a CXCL12-gradient. At 4h, migrated PBMCs were stained for CD5/CD19/CD3 and counted volumetrically by flow cytometry.
Results/
Conclusions
ODN 2006 induced heterogenous migratory changes in a cohort of 89 patient samples (ranging from 3.7 to 0.09-fold), which showed no correlation with the protein expression levels of either TLR9 or CXCR4 (CXCL12-receptor). We performed RNA sequencing on samples at either end of the migratory response spectrum to look for differences in the expression levels of downstream signaling components. We compared Responders (R) (i.e., migration increased >1.2-fold) vs Reverse Responders (RR) (i.e., migration decreased >0.8-fold) and found no transcriptional differences in TLR9, MYD88, IRAK4, IRAK1, IRAK2, TRAF6, or CXCR4. We then wanted to assess whether TLR9 signaling was inducible following ODN 2006-stimulation in both groups. At 24h post-stimulation, protein expression of IRAK1 was strongly degraded in R and RR alike, therefore confirming signal inducibility. IRAK4 expression however was significantly higher in R vs RR samples, suggesting an increased capacity for TLR-signaling in R samples. Following stimulation with ODN 2006, our RNA sequencing data identified significant upregulation of the NFκB-target genes: NFKBIA, NFKB2 and TNFAIP3 (which code for IκBα, P100 and A20 respectively) in both R and RR samples. Importantly, P100 was confirmed to upregulate at the protein level, and this upregulation correlated with the migratory response to ODN 2006; this suggested that the strength of TLR9-induced NFκB-signaling may influence migratory outcomes.
We hypothesized that TLR9-induced signaling may differ between patient samples due to heterogenous levels of basal BCR-signaling. Using in-silico modelling, we simulated TLR9 activation with low/high levels of basal BCR activation. TLR9 activation induced IκBα degradation and p65:p50 dimerization only when basal signaling was low; simulated cells with high basal signaling remained unresponsive. In keeping with these predictions, laboratory experiments showed RR samples to have significantly higher basal p-BTK and significantly lower IκBα, relative to R samples; this is indicative of higher basal BCR-NFκB activation in RR vs R samples. Additionally, RR samples were associated with markers of aggressive disease (i.e., U-CLL, CD49d/CD38 positivity), whilst R samples were associated with markers of indolent disease (i.e., M-CLL, CD49d/CD38 negativity); since poor prognosis CLL is known to signal strongly through the BCR signaling pathway (Stevenson et al., 2011), we hypothesized that aggressive and indolent CLL may be driven by distinct signaling pathways.
We then investigated the effects of BTK/TLR9-repression on migratory outcomes by stimulating samples with ODN 2006 ± ibrutinib ± ODN INH-18. In R samples, ODN 2006 induced an increase in CLL migration in both the absence and presence of ibrutinib; a combination of ibrutinib + ODN INH-18 resulted in a significant reduction in ODN 2006-stimulated migration (relative to ibrutinib-alone). In RR samples, ODN 2006 no longer induced a reduction in CLL migration in the presence of ibrutinib, and some samples instead showed an increase in CLL migration; this was indicative of a heightened responsiveness to TLR9 activation when BCR-signaling was repressed. Ibrutinib + ODN INH-18 induced a reduction in ODN 2006-stimulated migration in 5/8 RR samples. We therefore hypothesized that TLR9 may promote BTKi resistance in subsets of CLL patients by promoting migration in a BTK-independent manner. Early analyses of clinical response data suggest that TLR9 may be implicated in the depth of treatment responses to ibrutinib monotherapy in patients with poor prognosis CLL.
Keywords : Toll-like receptor 9, migration, ibrutinib
Please indicate how this research was funded. : Medical Research Council (MRC) and Brighton and Sussex Medical School
Please indicate the name of the funding organization.: Medical Research Council (MRC) and Brighton and Sussex Medical School