Authors
Deepa Sampath, PhD, Daisy Y. Díaz Rohena, PhD, Yu Zhou, PhD, Janani Ravikrishnan, PhD, Jyotsana Singh, PhD, Chaomei Liu, Andrew D. Mitchell, MS, John R. Sanchez II, BS, Charmelle D.Williams, MS, Trisha K. Wathan, BS1, Bailey Slawin, BS1, Kevin Bowman, BS, Yaxia Yuan, PhD , Peiyi Zhang, Wanyi Hu, Julio Alcantara-Montiel, MD, PhD, Guangrong Zheng, PhD, William G Wierda, MD, PhD1, Nitin Jain, MD, MMSc, Daohong Zhou, MD, Jennifer A. Woyach, MD.
Background
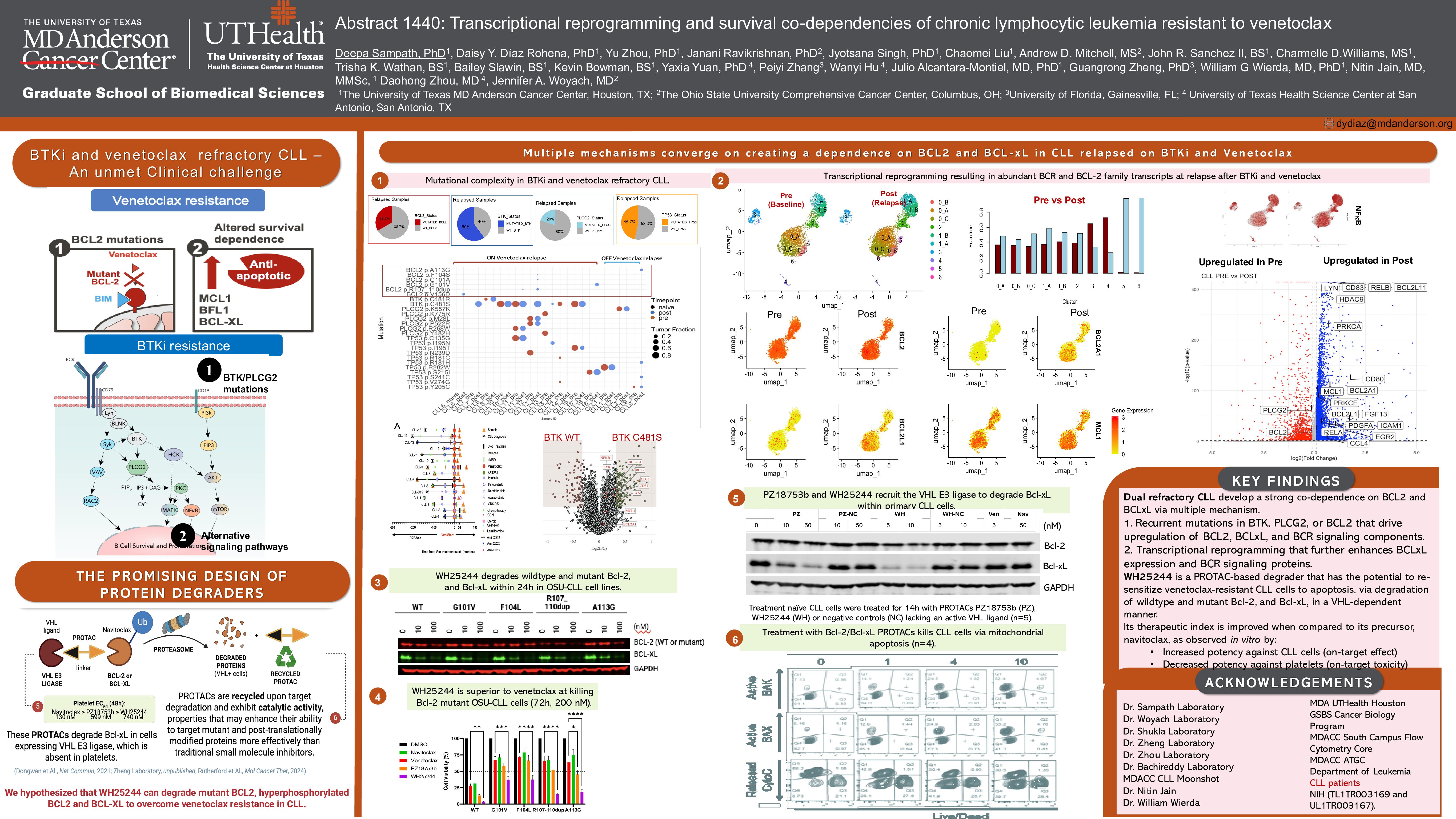
High-risk chronic lymphocytic leukemia (CLL) that progresses on both Bruton’s tyrosine kinase (BTK) inhibitors and venetoclax, particularly in the context of dysfunctional TP53, lacks effective treatment options. In a cohort of 15 dual-relapsed CLL, whole exome sequencing revealed that BCL2 mutations emerge in minor subclones at relapse, while TP53, BTK, and PLCG2 mutations dominate the relapsed population.
Results
Using single-cell RNA sequencing on paired pre-treatment and relapse samples, we observed significant transcriptional reprogramming impacting CLL survival. Among CLL cells (ROR1+/MS4A1+/CD19+/CD5+), we identified two minor relapse-specific subclusters (5 and 6) and three major clusters (0, 1, 2) present at both timepoints. Relapse-enriched clusters co-expressed BCL2, MCL1, and gained the MCL1-binding pro- apoptotic factor PMAIP1/NOXA, indicating a shift in apoptotic interactomes.
We also noted expansion of a pre-existing BCL2-low cluster (cluster 1), which gained NF-κB signaling, upregulated BCL2A1/BFL-1 and MCL1, and expressed high levels of p53 targets CDKN1A/p21 and GADD45A. This cluster expanded in patients with mutant TP53 at relapse (e.g., R282W, S215I), but not in wild-type or mutation-lost cases, suggesting mutant TP53 may drive this phenotype. The most striking case involved R282W-mutant CLL expanding post-treatment cessation, suggesting intrinsic resistance features. Meanwhile, most relapsed cells fell into clusters 0 and 2, which downregulated BCL2 and upregulated BCL2L1/BCL-XL.
Drug sensitivity profiling showed primary CLL cells progressed on venetoclax failed to activate pore- forming proteins BAK/BAX ( < 30% activation), despite maintaining BAK1 and BAX transcripts, and gaining BCL2L11/BIM, implying blockade of the apoptotic machinery. BH3 profiling confirmed functional co- dependence on BCL2 and alternative anti-apoptotic proteins, especially BCL-XL, as XXA1_Y4eK, a BCL-XL– specific BH3 peptide, triggered the strongest cytochrome C release.
To target this vulnerability, we tested WH25244, a novel BCL2/BCL-XL-targeting PROTAC, in 8 venetoclax- relapsed CLL samples. WH25244 is a bifunctional molecule derived from navitoclax that recruits VHL E3 ligases to BCL2 and BCL-XL proteins, resulting in degradation via the ubiquitin-proteasome system. The requirement of VHL for its activity allows WH25244 to target BCL2 / BCL-XL dependent cancer cells while sparing platelets from toxicity (BCL-XL dependent but VHL low). WH25244 effectively induced cytochrome C release and BAK/BAX activation (R2 = 0.7035, P = 0.0007). The highest sensitivity was observed in samples harboring BCL2 mutations (V156D, R107_110dup, G101V, F104S) and co-occurring TP53, BTK, and PLCG2 mutations. One highly sensitive sample had relapse-acquired BCL2L1 and BCR pathway genes (PLCG2, PRKCB, PRKCA).
WH25244 was validated in BCL-XL–dependent leukemia cell lines and in BCL2-mutant CRISPR knock-in CLL models (G101V, F104L, A113G, R107_110dup), where it degraded ~50% mutant BCL2 at 100 nM and 72 hr of treatment and induced more cell death than venetoclax. Structural modeling showed WH25244 forms stable ternary complexes with mutant BCL2 and VHL. Inactivation of the VHL E3 ligase ligand on WH25244 hampered its anti-leukemic effect and abrogated BCL-XL/BCL2 degradation. Importantly, WH25244 avoids platelet toxicity linked to BCL-XL inhibition, as platelets express minimal VHL. Last, we studied the effect of WH25244 against normal lymphocyte subsets from healthy donors. Apoptosis was detected at 18h of treatment by Annexin V / TMRM staining of CD19+ B cells, CD3+ T cells, and CD56+ NK cells via flow cytometry. Like venetoclax, WH25244 induced apoptosis of normal B cells in the nanomolar range. Toxicity to T cells remained low, and 100 nM WH25244 caused 1.7-fold more toxicity to NK cells than venetoclax.
Summary/Conclusion
These findings support WH25244 as a promising agent to overcome venetoclax resistance in dual BCL2/BTK inhibitor–relapsed CLL, by targeting resistant BCL2 variants and compensatory anti-apoptotic programs.
Keywords : CLL, Dual refractory CLL, transcriptional reprogramming
Please indicate how this research was funded. : Peer reviewed grant funding.
Please indicate the name of the funding organization.: CLL Global Research Foundation, University Of Texas MD Anderson CLL Moonshot, Leukemia Lymphoma Society – Translational research project.