Authors
Juliana Querol, Gabriel Fernández, Eugenia Payque, Rita Uría, Gimena Dos Santos, María Elena Márquez, Ana Inés Landoni, Victoria Irigoín, Pablo Muxi, Carolina Oliver, Gabriela de Galvez, Francis Kescherman, Raúl Gabus, Pablo Oppezzo, Nicholas Chiorazzi, Gerardo Ferrer, Florencia Palacios.
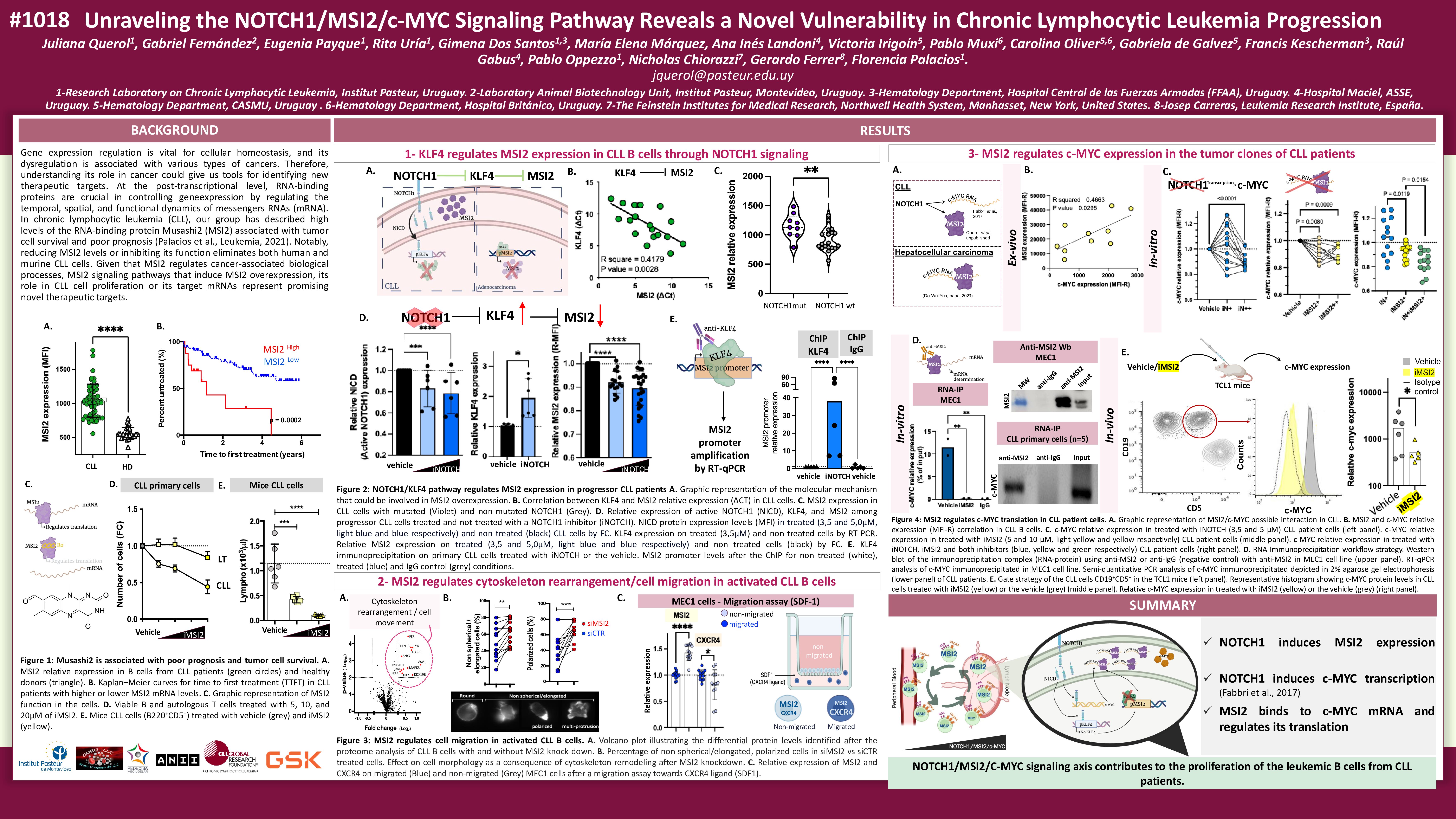
Gene expression regulation is vital for cellular homeostasis, and its dysregulation is associated with various types of cancers (Rosselló-Tortella, et al., 2020). At the post-transcriptional level, RNA-binding proteins are crucial in controlling gene expression by regulating the temporal, spatial, and functional dynamics of messenger RNAs (mRNA) (Pereira, et al., 2017). Among these proteins, the oncoprotein Musashi2 (MSI2) plays a significant role in these regulatory processes.
MSI2 regulates protein translation by binding to consensus sequences of target mRNAs. Interestingly, elevated MSI2 levels have been reported in various cancers, including chronic lymphocytic leukemia (CLL). Our group has identified that MSI2 promotes cell survival and tumor growth of CLL patient cells (Palacios, et al., Leukemia 2021). Later, we proposed a novel regulatory mechanism for MSI2, where the activation of the NOTCH1 signaling pathway induces MSI2 expression through the downregulation of a negative regulator of MSI2, Kruppel-like factor 4 (KLF4). Moreover, the reduction of MSI2 in NOTCH1 independent activated CLL cells results in an up-regulation of proteins associated with cell migration (Querol, et al., iwCLL 2023). Together, we propose that, in an activated tumor microenvironment, NOTCH1 signaling pathway induces MSI2 expression, and that increased MSI2 levels influence cell migration, likely retaining cells within proliferative niches where they receive survival signals and contribute to disease progression. Despite advances in MSI2 research, it is still unknown to which mRNAs MSI2 binds and regulates in B-lymphocytes from CLL cells.
Interestingly, in CLL, NOTCH1 signaling induces the transcription of the oncogene c-MYC (Fabbri et al., 2017), a transcription factor that regulates proliferation, apoptosis, differentiation, and metabolism. In addition, in acute myeloid leukemia the MSI2 regulatory pathway has been linked to c-MYC (Minuesa, et al., Nature 2019) and in hepatocellular carcinoma MSI2 has been shown to bind to c-MYC mRNA (Yeh et al., 2023). Based on these, we aimed to determine whether MSI2 regulates c-MYC translation in tumor clones of CLL patients. To answer this, we first determined MSI2/c-MYC protein expression levels in 10 B-CLL samples. Results showed a positive correlation between MSI2/c-MYC expression (r=0.47; p=0.029), where patients with poor outcomes show higher levels of both proteins.
For further investigation, B-cells from 13 CLL patients were treated in-vitro with either the MSI2 inhibitor Ro082750 (5 µM) or vehicle for 24 hours, and c-MYC protein levels were assessed by flow cytometry. The results showed that blocking MSI2 function reduced c-MYC protein levels in 12 of 13 samples (p=0.0042). Additionally, we examined the effect of the MSI2 inhibitor on c-MYC expression in TCL1 mice. Animals treated with the inhibitor showed a less number of tumoral cells and a reduction of c-MYC protein levels in CD19+CD5+ cells, further supporting the role of MSI2 in regulating c-MYC. Together, these findings support the interaction between MSI2 and c-MYC in CLL. To document that MSI2 directly binds to c-MYC mRNA, we performed RNA immunoprecipitation on primary cells from 5 CLL samples using an anti-MSI2 antibody and the anti-isotype IgG as negative control. After retro-transcription, c-MYC was amplified by PCR, confirming that MSI2 regulates c-MYC translation by binding to its mRNA in CLL samples.
Moreover, because NOTCH1 activates the expression of survival genes, including the transcription of c-MYC, we wonder whether blocking NOTCH1 and MSI2 signaling could enhance c-MYC reduction. To address this, CLL samples (n=13) were treated with both NOTCH1 (DAPT, gamma-secretase inhibitor) and MSI2 (Ro082750) inhibitors. The results showed that blocking NOTCH1 and MSI2 signaling led to a greater reduction of c-MYC levels than a single inhibitor in CLL cells (p≤0.0001), suggesting a potential strategy for reducing tumor cell viability.
In this work, we propose that the NOTCH1/MSI2/C-MYC signaling axis contributes to the proliferation of the leukemic B cells from CLL patients. NOTCH1 induces MSI2 expression by downregulating KLF4 and promotes the transcription of c-MYC. In addition, we observed that MSI2 binds to c-MYC mRNA and positively regulates its translation.
Our results provide insights into the regulation of c-MYC in B-cells from CLL patients and suggests that disrupting the NOTCH1/MSI2/c-MYC axis could offer a different targeted therapeutic strategy for certain patients. Confirmatory studies are crucial, and may open new avenues for highly targeted CLL therapies.
Keywords : Notch1 Musashi2c-MYC
Please indicate how this research was funded. : The grant was founded by the national agency of research and innovation from Uruguay and more recently by the CLL Global research foundation.
Salary from the principal investigator was supported by the Institute Pasteur Montevideo, Uruguay and the first author has a fellowship from the national agency.
Please indicate the name of the funding organization.: National Agency (Agencia Nacional de investigación e innovación), Uruguay
CLL Global Research Foundation