Authors
Francesco Angotzi, Raffaella Pasquale, Massimo Moratti, Luca Laurenti, Tommaso Quaranta, Idanna Innocent, Gioachino Catania, Massimo Gentile, Marta Coscia, Giulia Zamprogna, Enrico Lista, Paolo Sportoletti, Alberto Fresa, Andrea Galitzia, Valerio Guarente, Alessandro Cellini, Arianna Bevilacqua, Chiara Adele Cavarretta, Andrea Serafin, Marco Pizzi, Enrico Gaffo, Stefania Bortoluzzi, Livio Trentin, Andrea Visentin.
Introduction
Accelerated chronic lymphocytic leukemia (aCLL) is a rare and understudied form of chronic lymphocytic leukemia (CLL) with aggressive histological features, considered to fall biologically between typical CLL and Richter’s transformation (RT). We conducted a multicenter retrospective study to shed light on the clinical and biological characteristics of aCLL in relation to CLL and RT.
Methods
We enrolled patients with a histologically proven diagnosis of aCLL according to the criteria published by Giné et. al in 2010. Clinical and biological information were extracted from the medical records and pathology reports across participating institutions. Two cohorts of patients with histologically proven diagnoses of CLL and RT treated at the University Hospital of Padua were enrolled as control groups in order to compare them with aCLL patients. Moreover, we performed NGS analysis using a 108-gene panel (Sophia lymphoma solution by NextSeq) and scRNA-seq (at least 10.000 nuclei/sample, 150 bases per end by NovaSeq) on FFPE lymph-node tissues from 12 patients (n=7 aCLL, n=2 RT, n=3 CLL) and 16 patients (n=10 aCLL, n=3 RT, n=3 CLL) respectively. For time-to-event outcomes, overall survival (OS) was calculated as the time from aCLL diagnosis until death or last follow-up, and time-to-next treatment from the time at which the first therapy for aCLL was started until the next line of therapy or death.
Results
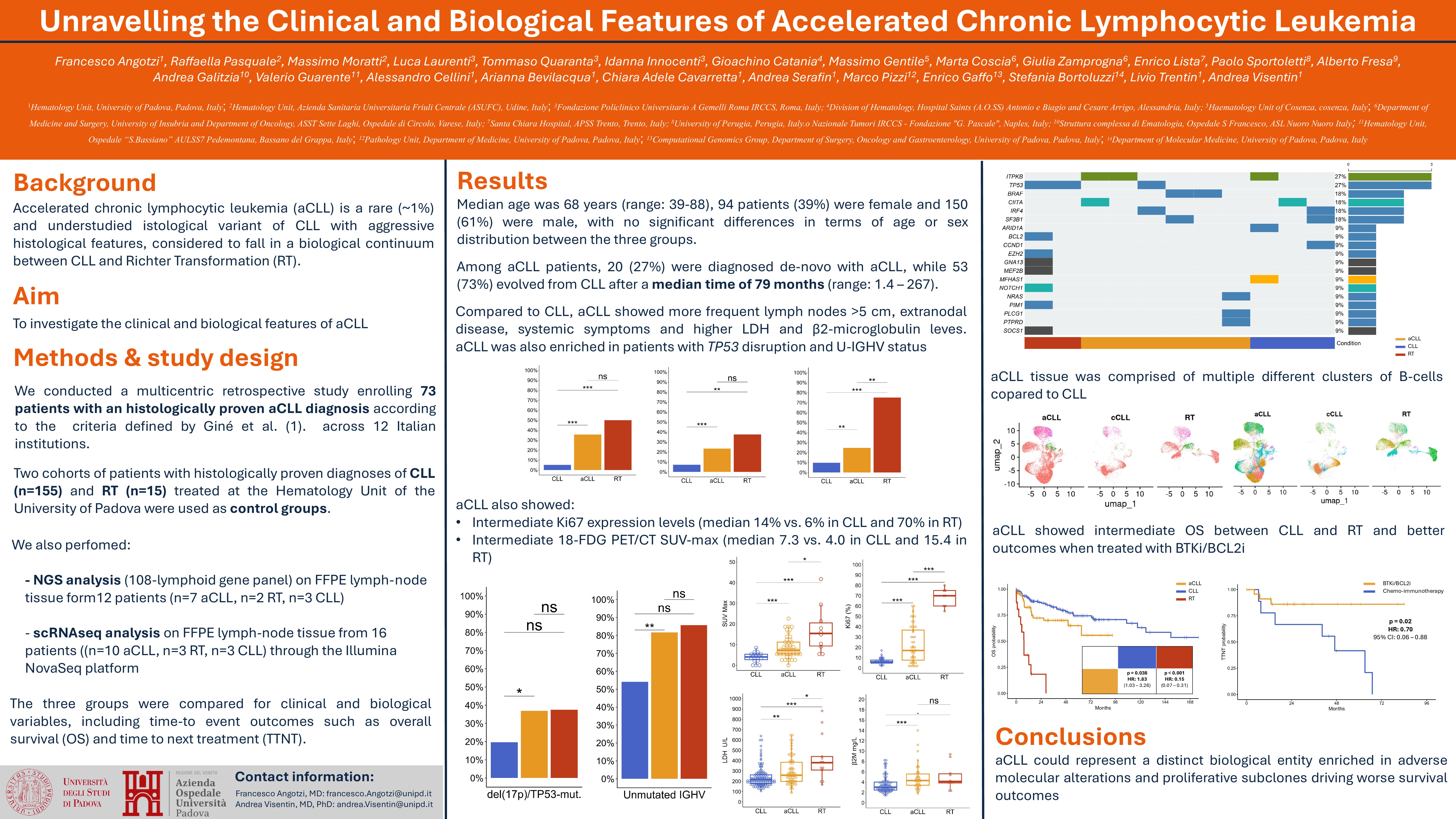
Among 244 patients enrolled, 73 (30%) had aCLL, 16 (7%) RT, 155 (63%) CLL. Median age was 68 years (range: 39-88), 94 patients (39%) were female and 150 (61%) were male, with no significant differences in terms of age or sex distribution between the three groups. Among aCLL patients, 20 (27%) were diagnosed de-novo with aCLL, while 53 (73%) evolved from CLL after a median time of 79 months (range: 1.4 – 267).
Compared to CLL, aCLL patients more frequently presented extranodal involvement (27% vs. 8%; p< 0.001), lymphadenopathies >5cm (41% vs 5%; p < 0.001), constitutional symptoms (26% vs 10%; p=0.002), and had a higher CLL-IPI scores (median 5 vs 4; p=0.02).
A total of 77 (32%) patients also had PET/CT data available, with aCLL displaying a higher SUVmax than CLL (median 7.3 vs. 4.0; p< 0.001), but still lower than in RT (median 15.4; p=0.01). Similarly, Ki-67 expression was higher in aCLL than CLL (median 14% vs. 6%; p< 0.001) but much lower than in RT (70%; p< 0.001).
Both aCLL and RT were enriched in patients with unmutated IGHV status (81% and 86% respectively vs. 54%; p< 0.001), and aCLL also had a significantly higher frequency of TP53 disruption (del17p and/or TP53 mutations) (36% vs. 20%; p=0.01), and a higher median number of karyotype abnormalities (3 vs. 1; p=0.001) compared to CLL. There were instead no differences between the three groups regarding the frequency of del(11q), del(13q) and trisomy 12. RT patients had the higher rate of NOTCH1 mutations (44% vs. 31% in aCLL and 12% in CLL; p= 0.03).
NGS analysis of aCLL lymph-node tissue revealed recurrent mutations in TP53, BRAF, and ITPKB genes (each one present in 16% of the analyzed patients), but also in CIITA, IRF4, SF3B1, NRAS, PLCG1, and PTPRD. scRNA-seq data demonstrated that aCLL samples were enriched in cells expressing a proliferative transcriptional signature, likely corresponding to histologic proliferation centers.
The OS of patients with aCLL was significantly shorter than CLL patients (5-year OS 64% vs. 74%; p=0.03) but longer than those with RT (median 7.5 months; p < 0.001). Among aCLL patients, 48 (66%) were treated with targeted agents (n=28 BTKi, n=11 venetoclax, n=7 ibrutinib-venetoclax, n=1 BTK-degraders, n=1 idelalisib), 13 (18%) received chemoimmunotherapy (CIT), while 12 (16%) required no treatment. Patients with aCLL receiving first line treatment with targeted agents experienced longer TTNT compared with those receiving CIT (median NR vs. 47 months; p=0.02).
Conclusion
This multicenter study highlights aCLL as a clinically and biologically distinct, aggressive entity with features intermediate between CLL and RT. aCLL is enriched in adverse molecular alterations and proliferative subclones, contributing to its distinct histology and inferior outcomes relative to CLL. Our findings also suggest that targeted therapies may provide superior disease control compared with CIT in aCLL, warranting further prospective validation.
Keywords : Accelerated Chronic Lymphocytic Leukemia, singe-cell RNA sequencing, NGS
Please indicate how this research was funded. : N/A
Please indicate the name of the funding organization.: N/A