Authors
Isabel Paulos Mesquita, Margarida Duarte, Ana Vagos Mata, Ana Amarante, Sérgio Chacim, Sara Duarte, André Airosa Pardal, Patrícia Rocha Silva, Ana Rebelo, Inês Vieira, Mariana Trigo Miranda, Inês Sá, Filipe Pinto, Bruno Mesquita, Cátia Sol dos Reis, Teresa Ribeiro, Sofia Ramalheira, João Paulo Fernandes, Renata Cabral, Mónica Santos, Joana Caldas, Ana Luísa Pinto, Rita Coutinho, Daniela Alves.
Introduction and methods
Rituximab-Venetoclax (RV) has become the standard of care for relapsed/refractory Chronic Lymphocytic Leukemia (CLL), following its demonstrated superiority over chemoimmunotherapy (CIT) in the phase III MURANO trial. This has led to the approval of this combination in Europe in 2018 for patients with previously treated CLL. Robust population-level data reflecting routine clinical practice is lacking. Therefore, on behalf of the Portuguese Lymphoma Group (GPL), we conducted a multicenter retrospective analysis across 12 Portuguese centers, including patients treated with RV from 2019 to 2023, with a data cut off on April 30, 2025. The primary endpoints were progression-free survival (PFS) and overall survival (OS). As secondary endpoints we report time to next treatment or death (TTNTD), response rates, feasibility and safety outcomes, assessed per iwCLL criteria.
Results
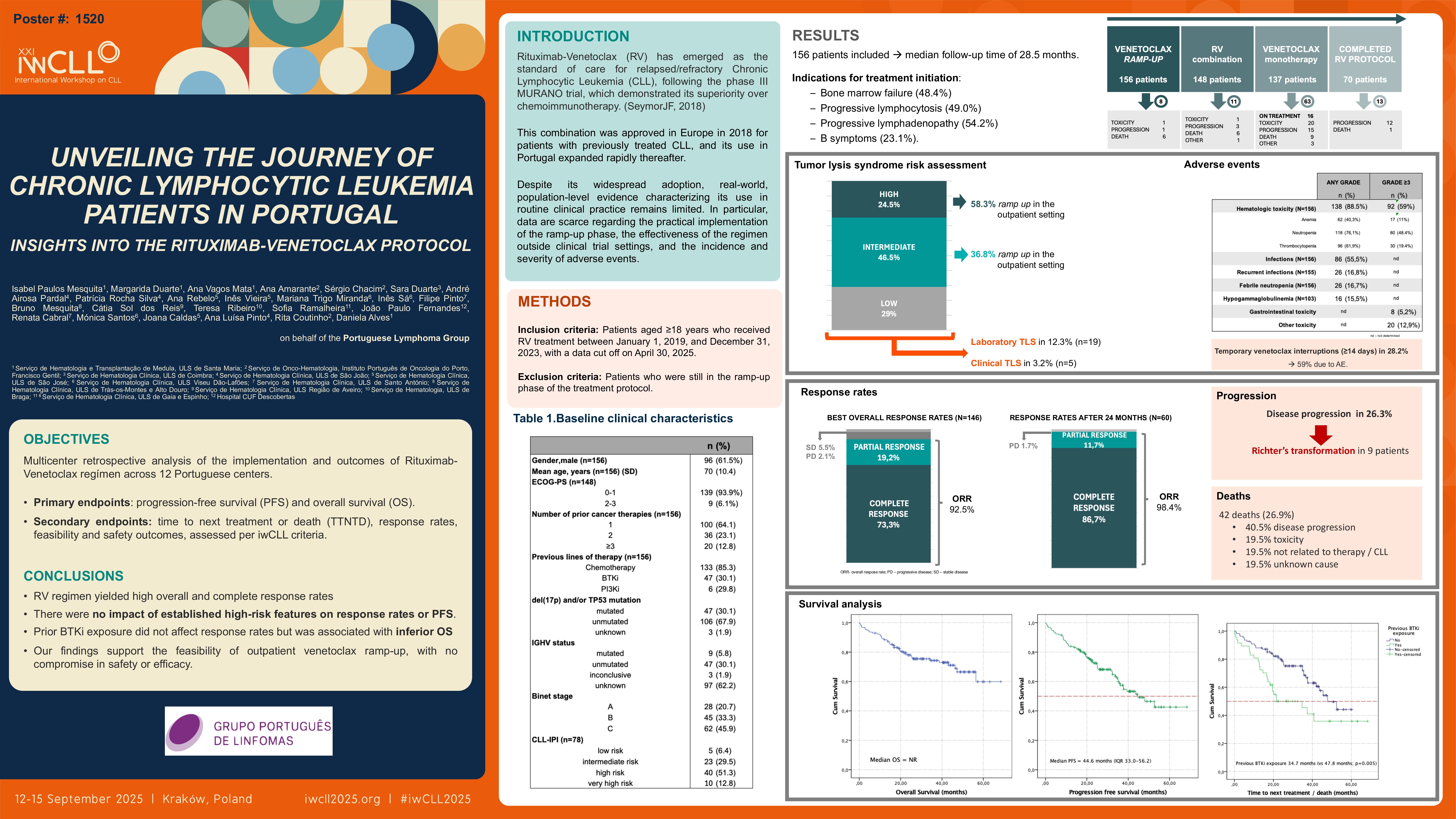
A total of 156 patients was included, with a median age of 70 years (IQR: 62.3–75); 61.5% were male and 93.9% had ECOG ≤1. Unfavorable genetic features were observed in a substantial subset, with mutated TP53 and/or del(17p) in 30.1%. Most patients had previously received CIT (85.3%) and 30.1% had already been exposed to BTK inhibitors (BTKi). Indications for treatment initiation included bone marrow failure (48.4%), lymphocytosis (49.0%), lymphadenopathy (54.2%), and B symptoms (23.1%).
92 patients (58.9%) underwent venetoclax ramp-up entirely in the outpatient setting, with a median of 6 appointments during this period. Although tumor lysis syndrome (TLS) risk estimates were intermediate or high in 71% of the patients, laboratory and clinical TLS were observed in only 12.3% and 3.2%, respectively. At the time of analysis, 20 patients remained on treatment, while 70 had completed the 24-month protocol. Permanent early discontinuation occurred in 66 pts due to disease progression (30.3%), toxicity (33.3%), death (30.3%) or other causes (7.5%). Hematologic toxicity was the most frequently observed adverse event (AE), with grade ≥3 cytopenias in 59% of patients: neutropenia (51.6%), thrombocytopenia (20.5%), and anemia (10.9%). Febrile neutropenia was reported in 16.7%, and recurrent infections in 17.9%, most of which occurred during or following the venetoclax monotherapy phase. Grade ≥3 gastrointestinal toxicity was observed in 5.1%. Temporary venetoclax interruptions (≥14 days) were required in 28.2% of patients, predominantly due to AE.
The overall response rate was 86.5%, with 68.6% achieving complete responses. For those who completed the 24mo treatment protocol (n=70), response was assessed in 60 patients with 86.7% presenting complete response. Disease progression (PD) was documented in 26.3%, 9 of them with Richter’s transformation upon PD. With a median follow-up of 28.5 months, the median OS was not reached, while median PFS and TTNTD were 44.6 and 45.5 months, respectively. No baseline clinical or biological features, including known high-risk factors, were found to significantly impact response rates or PFS. OS was impacted by prior exposure to BKTi (46.4 months vs NR; p = 0.015). The subgroup analysis of these patients (of which 57% presented del17p/TP53) also revealed significantly inferior TTNTD (34.7 vs 47.8 months; p=0.005), with no difference on PFS (33.6 vs 47.9 months; p=0.081). Deaths were registered in 26.9% – 39% due to PD, 19.5% due to toxicity, 19.5% due to causes non CLL or treatment related and 19.5% due to unknown causes.
Conclusion
In this real-world cohort, the RV regimen yielded high overall and complete response rates, with no apparent impact of established high-risk features on response or PFS. Although prior BTKi exposure did not affect response rates, it was associated with inferior OS, underscoring the need for further investigation into optimal sequencing strategies for this subgroup. Additionally, our findings support the feasibility of outpatient venetoclax ramp-up, with no compromise in safety or efficacy.
Keywords : CLL, Relapsed/refractory
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: