Authors
Andrea Serafin, Francesco Angotzi1, Alessandro Cellini, Arianna Bevilacqua, Nicolo’ Danesin, Annalisa Martines, Laura Bonaldi, Riccardo Moia, Gianluca Gaidano, Alessandro Noto, Sara Pepe, Francesca Cibien, Maria Ilaria Del Principe, Gianmatteo Rigolin, Antonio Cuneo, Anna Maria Frustaci, Alessandra Tedeschi, Francesca Romana Mauro, Lydia Scarfò, Paolo Ghia, Livio Trentin, Andrea Visentin.
Background
Novel targeted therapies have transformed the treatment landscape of chronic lymphocytic leukemia (CLL), with Bruton tyrosine kinase inhibitors (BTKi) and BCL2 inhibitors showing remarkable efficacy. However, their long-term impact on genomic evolution – particularly regarding cytogenetic complexity and clonal dynamics – remains poorly understood. Kittai et al. retrospectively analyzed CLL patients treated with ibrutinib and found that cytogenetic evolution independently predicted poor outcomes at disease progression (PD). Among 75 patients, 56% showed an increase in chromosomal abnormalities, and 80% displayed karyotypic evolution at PD. However, the cohort’s heterogeneous treatment history limited therapy-specific interpretation. In contrast, Fürstenau et al. evaluated venetoclax-based regimens and observed relative cytogenetic stability, though the small sample size (n=20) precludes firm conclusions. Together, these studies highlight both the clinical relevance and the current gaps in understanding cytogenetic evolution under targeted therapies in CLL.
Aim
To compare the frequency and dynamics of cytogenetic evolution in treatment naïve patients receiving continuous BTKi therapy versus those receiving fixed-duration Ven-based regimens.
Methods
We conducted a retrospective, multicenter cohort study involving patients with CLL who underwent treatment with targeted therapies from 2015 to 2024. Not all participating centers have yet completed data submission, and analyses presented here reflect an interim dataset. An initial cohort of 834 patients who had undergone cytogenetic analysis was screened. In the final analysis we included only treatment naïve patients who received a targeted agent and of whom conventional karyotyping data were available both before treatment initiation and at the time of PD. Complex karyotype (CK) was defined by the presence of at least 3 chromosomal alterations in the neoplastic clone (low-CK=3-4 alterations, high-CK=5 or more alterations). Cytogenetic evolution was defined as either an increase in the number of chromosomal abnormalities or the acquisition of a new clone at PD.
Results
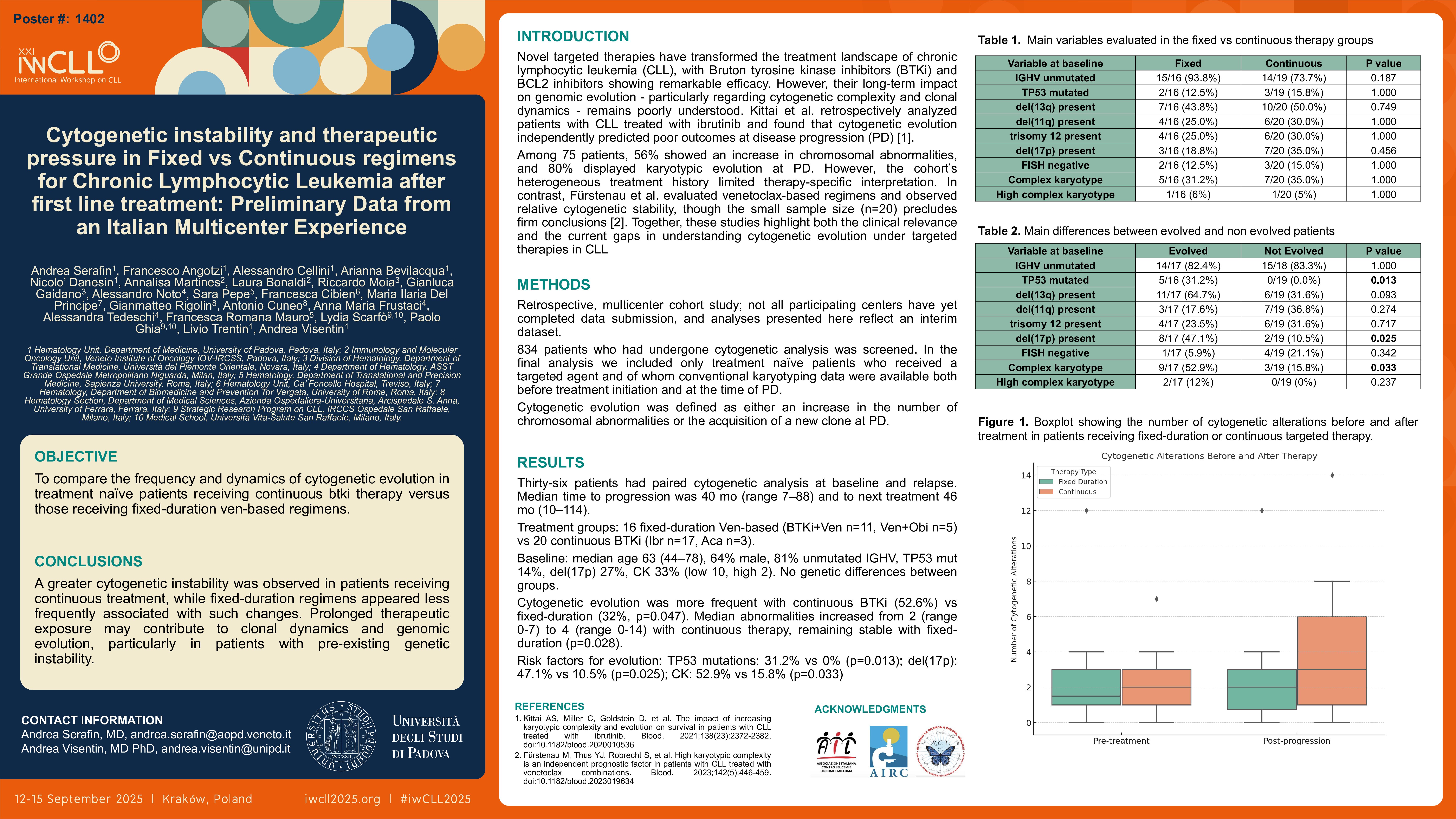
Among relapsed patients paired cytogenetic analysis at baseline and at relapse was successfully performed in 36 patients. Median time to progression was 40 months (range 7-88) and median time to next treatment was 46 months (range 10-114). Sixteen patients received fixed-duration venetoclax-based therapy (BTKi+Ven n=11, Ven+obinutuzumab n=5) and 20 received continuous BTKi (ibrutinib n=17, acalabrutinib n=3). At baseline, median age was 63 (range 44-78), 23 (64%) were males, 81% (n=29) had unmutated IGHV, 14% (n=5) had TP53 mutations 27% (n=10) had del(17p), and 33% (n=12) complex karyotype (low-CK n=10, high-CK n=2). No differences in terms of genetic alterations were observed among the two groups (p>0.05). Cytogenetic evolution was significantly more frequent in the continuous group (52.6%) compared to the fixed-duration group (32%, p = 0.047). The median number of cytogenetic abnormalities increased in the continuous group from 2 (range: 0–5) before treatment to 4 (range: 0–8) at progression, while it remained stable in the fixed-duration group (median 2 pre and post). The difference in alterations between pre- and post-treatment was significantly higher in the continuous group (p = 0.028). Four patients with non-CK evolved to low-CK while 2 non-CK and 5 low-CK patients became high-CK. 18 out of 24 non-CK patients and 4 out of 12 CK patients remain stable at PD.
Importantly, the presence of TP53 mutations, del(17p), and baseline complex karyotype were all significantly associated with cytogenetic evolution. TP53 mutations were observed in 31.2% of evolving cases versus 0% in non-evolving cases (p = 0.013), while del(17p) was present in 47.1% of evolving patients compared to only 10.5% in the non-evolving group (p = 0.025). Complex karyotype at baseline was detected in 52.9% of evolving cases versus 15.8% of non-evolving patients (p = 0.033).
Conclusions
These preliminary findings suggest that both the type of targeted therapy and the presence of specific baseline genetic features may influence the likelihood of cytogenetic evolution during the clinical course of CLL. A greater cytogenetic instability was observed in patients receiving continuous treatment, while fixed-duration regimens appeared less frequently associated with such changes. These data raise the hypothesis that prolonged therapeutic exposure may contribute to clonal dynamics and genomic evolution, particularly in patients with pre-existing genetic instability. This underscores the potential relevance of integrating cytogenetic profiling into longitudinal disease monitoring strategies, especially in those patients with a high-risk genetic disease.
Keywords : CLL, clonal-evolution, karyotyping
Please indicate how this research was funded. :
Please indicate the name of the funding organization.: